Tumor-derived microvesicles promote regulatory T cell expansion and induce apoptosis in tumor-reactive activated CD8+ T lymphocytes
- PMID: 19692638
- PMCID: PMC3721354
- DOI: 10.4049/jimmunol.0900970
Tumor-derived microvesicles promote regulatory T cell expansion and induce apoptosis in tumor-reactive activated CD8+ T lymphocytes
Abstract
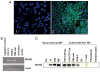
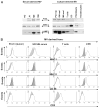
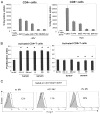
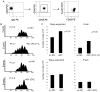
Sera of patients with cancer contain membraneous microvesicles (MV) able to induce apoptosis of activated T cells by activating the Fas/Fas ligand pathway. However, the cellular origin of MV found in cancer patients' sera varies as do their molecular and cellular profiles. To distinguish tumor-derived MV in cancer patients' sera, we used MAGE 3/6(+) present in tumors and MV. Molecular profiles of MAGE 3/6(+) MV were compared in Western blots or by flow cytometry with those of MV secreted by dendritic cells or activated T cells. These profiles were found to be distinct for each cell type. Only tumor-derived MV were MAGE 3/6(+) and were variably enriched in 42-kDa Fas ligand and MHC class I but not class II molecules. Effects of MV on signaling via the TCR and IL-2R and proliferation or apoptosis of activated primary T cells and T cell subsets were also assessed. Functions of activated CD8(+) and CD4(+) T lymphocytes were differentially modulated by tumor-derived MV. These MV inhibited signaling and proliferation of activated CD8(+) but not CD4(+) T cells and induced apoptosis of CD8(+) T cells, including tumor-reactive, tetramer(+)CD8(+) T cells as detected by flow cytometry for caspase activation and annexin V binding or by DNA fragmentation. Tumor-derived but not dendritic cell-derived MV induced the in vitro expansion of CD4(+)CD25(+)FOXP3(+) T regulatory cells and enhanced their suppressor activity. The data suggest that tumor-derived MV induce immune suppression by promoting T regulatory cell expansion and the demise of antitumor CD8(+) effector T cells, thus contributing to tumor escape.
Conflict of interest statement
The authors have no financial conflict of interest.
Figures


References
-
- Denzer K, Kleijmeer MJ, Heijnen HF, Stoorvogel W, Gellze HJ. Exosome: from internal vesicle of the multivesicular body to intercellular signaling device. J Cell Sci. 2000;113:3365–3374. - PubMed
-
- Johnstone RM. Exosomes biological significance: a concise review. Blood Cells Mol Dis. 2006;36:315–321. - PubMed
-
- van Niel G, Porto-Carreiro I, Simors S, Raposo G. Exosomes: a common pathway for a specialized function. J Biochem. 2006;140:13–21. - PubMed
-
- Ratajczak J, Wysoczynski M, Hayek F, Janowska-Wieczorek A, Ratajczak MZ. Membrane-derived microvesicles: important and underappreciated mediators of cell-to-cell communication. Leukemia. 2006;20:1487–1495. - PubMed
-
- Martinez-Lorenzo MJ, Anel A, Gamen S, Monle NI, Lasierra P, Larrad L, Pineiro A, Alava MA, Naval J. Activated human T cells release bioactive Fas ligand and APO2 lignad in microvesicles. J Immunol. 1999;163:1274–1281. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

