Letrozole therapy alone or in sequence with tamoxifen in women with breast cancer
- PMID: 19692688
- PMCID: PMC2921823
- DOI: 10.1056/NEJMoa0810818
Letrozole therapy alone or in sequence with tamoxifen in women with breast cancer
Abstract
Background: The aromatase inhibitor letrozole, as compared with tamoxifen, improves disease-free survival among postmenopausal women with receptor-positive early breast cancer. It is unknown whether sequential treatment with tamoxifen and letrozole is superior to letrozole therapy alone.
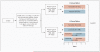
Methods: In this randomized, phase 3, double-blind trial of the treatment of hormone-receptor-positive breast cancer in postmenopausal women, we randomly assigned women to receive 5 years of tamoxifen monotherapy, 5 years of letrozole monotherapy, or 2 years of treatment with one agent followed by 3 years of treatment with the other. We compared the sequential treatments with letrozole monotherapy among 6182 women and also report a protocol-specified updated analysis of letrozole versus tamoxifen monotherapy in 4922 women.
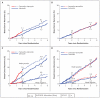
Results: At a median follow-up of 71 months after randomization, disease-free survival was not significantly improved with either sequential treatment as compared with letrozole alone (hazard ratio for tamoxifen followed by letrozole, 1.05; 99% confidence interval [CI], 0.84 to 1.32; hazard ratio for letrozole followed by tamoxifen, 0.96; 99% CI, 0.76 to 1.21). There were more early relapses among women who were assigned to tamoxifen followed by letrozole than among those who were assigned to letrozole alone. The updated analysis of monotherapy showed that there was a nonsignificant difference in overall survival between women assigned to treatment with letrozole and those assigned to treatment with tamoxifen (hazard ratio for letrozole, 0.87; 95% CI, 0.75 to 1.02; P=0.08). The rate of adverse events was as expected on the basis of previous reports of letrozole and tamoxifen therapy.
Conclusions: Among postmenopausal women with endocrine-responsive breast cancer, sequential treatment with letrozole and tamoxifen, as compared with letrozole monotherapy, did not improve disease-free survival. The difference in overall survival with letrozole monotherapy and tamoxifen monotherapy was not statistically significant. (ClinicalTrials.gov number, NCT00004205.)
2009 Massachusetts Medical Society
Figures


Comment in
-
Aromatase inhibitors alone or in sequence with tamoxifen - clinical evaluation of the BIG 1-98 trial.Expert Opin Pharmacother. 2010 Feb;11(3):489-92. doi: 10.1517/14656560903501551. Expert Opin Pharmacother. 2010. PMID: 20053139 Clinical Trial. No abstract available.
References
-
- Early Breast Cancer Trialists’ Collaborative Group Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365:1687–717. - PubMed
-
- The Breast International Group (BIG) 1-98 Collaborative Group A comparison of letrozole and tamoxifen in postmenopausal women with early breast cancer. N Engl J Med. 2005;353:2747–57. - PubMed
-
- Coates AS, Keshaviah A, Thürlimann B, et al. Five years of letrozole compared with tamoxifen as initial adjuvant therapy for postmenopausal women with endocrine-responsive early breast cancer: update of study BIG 1-98. J Clin Oncol. 2007;25:486–92. - PubMed
-
- Forbes JF, Cuzick J, Buzdar A, Howell A, Tobias JS, Baum M. Effect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 100-month analysis of the ATAC trial. Lancet Oncol. 2008;9:45–53. - PubMed
-
- Baum M, Budzar AU, Cuzick J, et al. Anastrozole alone or in combination with tamoxifen versus tamoxifen alone for adjuvant treatment of postmenopausal women with early breast cancer: first results of the ATAC randomised trial. Lancet. 2002;359:2131–9. Erratum, Lancet 2002;360:1520. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
