Foamy macrophages and the progression of the human tuberculosis granuloma
- PMID: 19692995
- PMCID: PMC2759071
- DOI: 10.1038/ni.1781
Foamy macrophages and the progression of the human tuberculosis granuloma
Abstract
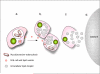
The progression of tuberculosis from a latent, subclinical infection to active disease that culminates in the transmission of infectious bacilli is determined locally at the level of the granuloma. This progression takes place even in the face of a robust immune response that, although it contains infection, is unable to eliminate the bacterium. The factors or environmental conditions that influence this progression remain to be determined. Recent advances have indicated that pathogen-induced dysregulation of host lipid synthesis and sequestration serves a critical role in this transition. The foamy macrophage seems to be a key participant in both sustaining persistent bacteria and contributing to the tissue pathology that leads to cavitation and the release of infectious bacilli.
Figures

References
-
- Parrish NM, Dick JD, Bishai WR. Mechanisms of latency in Mycobacterium tuberculosis. Trends Microbiol. 1998;6:107–112. - PubMed
-
- Rohde K, Yates RM, Purdy GE, Russell DG. Mycobacterium tuberculosis and the environment within the phagosome. Immunol Rev. 2007;219:37–54. - PubMed
-
- Sturgill-Koszycki S, et al. Lack of acidification in Mycobacterium phagosomes produced by exclusion of the vesicular proton-ATPase. Science. 1994;263:678–81. - PubMed
-
- Gohn A, editor. Der primäre Lungenherd bei der Tuberkulose der Kinder (The Primary Lung Lesion in Infant TB) Berlin: 1912.
-
- Saunders BM, Cooper AM. Restraining mycobacteria Role of granulomas in mycobacterial infections. Immunol Cell Biol. 2000;78:334–341. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

