Tumor invasion of Salmonella enterica serovar Typhimurium is accompanied by strong hemorrhage promoted by TNF-alpha
- PMID: 19693266
- PMCID: PMC2724709
- DOI: 10.1371/journal.pone.0006692
Tumor invasion of Salmonella enterica serovar Typhimurium is accompanied by strong hemorrhage promoted by TNF-alpha
Abstract
Background: Several facultative anaerobic bacteria with potential therapeutic abilities are known to preferentially colonize solid tumors after systemic administration. How they efficiently find and invade the tumors is still unclear. However, this is an important issue to be clarified when bacteria should be tailored for application in cancer therapy.
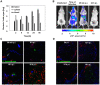
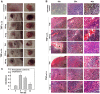
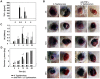
Methodology/principal findings: We describe the initial events of colonization of an ectopic transplantable tumor by Salmonella enterica serovar Typhimurium. Initially, after intravenous administration, bacteria were found in blood, spleen, and liver. Low numbers were also detected in tumors associated with blood vessels as could be observed by immunohistochemistry. A rapid increase of TNF-alpha in blood was observed at that time, in addition to other pro-inflammatory cytokines. This induced a tremendous influx of blood into the tumors by vascular disruption that could be visualized in H&E stainings and quantified by hemoglobin measurements of tumor homogenate. Most likely, together with the blood, bacteria were flushed into the tumor. In addition, blood influx was followed by necrosis formation, bacterial growth, and infiltration of neutrophilic granulocytes. Depletion of TNF-alpha retarded blood influx and delayed bacterial tumor-colonization.
Conclusion: Our findings emphasize similarities between Gram-negative tumor-colonizing bacteria and tumor vascular disrupting agents and show the involvement of TNF-alpha in the initial phase of tumor-colonization by bacteria.
Conflict of interest statement
Figures



References
-
- Coley WB. The treatment of malignant tumors by repeated inoculations of erysipelas. With a report of ten original cases. 1893. Clin Orthop Relat Res. 1991:3-11. - PubMed
-
- Van Mellaert L, Barbe S, Anne J. Clostridium spores as anti-tumour agents. Trends Microbiol. 2006;14:190–196. - PubMed
-
- Pawelek JM, Low KB, Bermudes D. Bacteria as tumour-targeting vectors. Lancet Oncol. 2003;4:548–556. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

