Collagens
- PMID: 19693541
- PMCID: PMC2997103
- DOI: 10.1007/s00441-009-0844-4
Collagens
Abstract
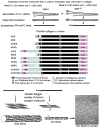
The collagens represent a family of trimeric extracellular matrix molecules used by cells for structural integrity and other functions. The three alpha chains that form the triple helical part of the molecule are composed of repeating peptide triplets of glycine-X-Y. X and Y can be any amino acid but are often proline and hydroxyproline, respectively. Flanking the triple helical regions (i.e., Col domains) are non-glycine-X-Y regions, termed non-collagenous domains. These frequently contain recognizable peptide modules found in other matrix molecules. Proper tissue function depends on correctly assembled molecular aggregates being incorporated into the matrix. This review highlights some of the structural characteristics of collagen types I-XXVIII.
Figures



References
-
- Anderson S, SundarRaj S, Fite D, Wessel H, SundarRaj N. Developmentally regulated appearance of spliced variants of type XII collagen in the cornea. Invest Ophthalmol Vis Sci. 2000;41:55–63. - PubMed
-
- Aumailley M, Has C, Tunggal L, Bruckner-Tuderman L. Molecular basis of inherited skin-blistering disorders, and therapeutic implications. Expert Rev Mol Med. 2006;8:1–21. - PubMed
-
- Bader HL, Keene DR, Charvet B, Veit G, Driever W, Koch M, Ruggiero F. Zebrafish collagen XII is present in embryonic connective tissue sheaths (fascia) and basement membranes. Matrix Biol. 2009;28:32–43. - PubMed
-
- Banyard J, Bao L, Zetter BR. Type XXIII collagen, a new transmembrane collagen identified in metastatic tumor cells. J Biol Chem. 2003;278:20989–20994. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

