Structure of PqsD, a Pseudomonas quinolone signal biosynthetic enzyme, in complex with anthranilate
- PMID: 19694421
- PMCID: PMC2775144
- DOI: 10.1021/bi9009055
Structure of PqsD, a Pseudomonas quinolone signal biosynthetic enzyme, in complex with anthranilate
Abstract
Pseudomonas quinolone signal (PQS), 2-heptyl-3-hydroxy-4-quinolone, is an intercellular alkyl quinolone signaling molecule produced by the opportunistic pathogen Pseudomonas aeruginosa. Alkyl quinolone signaling is an atypical system that, in P. aeruginosa, controls the expression of numerous virulence factors. PQS is synthesized from the tryptophan pathway intermediate, anthranilate, which is derived either from the kynurenine pathway or from an alkyl quinolone specific anthranilate synthase encoded by phnAB. Anthranilate is converted to PQS by the enzymes encoded by the pqsABCDE operon and pqsH. PqsA forms an activated anthraniloyl-CoA thioester that shuttles anthranilate to the PqsD active site where it is transferred to Cys112 of PqsD. In the only biochemically characterized reaction, a condensation then occurs between anthraniloyl-PqsD and malonyl-CoA or malonyl-ACP, a second PqsD substrate, forming 2,4-dihydroxyquinoline (DHQ). The role PqsD plays in the biosynthesis of other alkyl quinolones, such as PQS, is unclear, though it has been reported to be required for their production. No evidence exists that DHQ is a PQS precursor, however. Here we present a structural and biophysical characterization of PqsD that includes several crystal structures of the enzyme, including that of the PqsD-anthranilate covalent intermediate and the inactive Cys112Ala active site mutant in complex with anthranilate. The structure reveals that PqsD is structurally similar to the FabH and chalcone synthase families of fatty acid and polyketide synthases. The crystallographic asymmetric unit contains a PqsD dimer. The PqsD monomer is composed of two nearly identical approximately 170-residue alphabetaalphabetaalpha domains. The structures show anthranilate-liganded Cys112 is positioned deep in the protein interior at the bottom of an approximately 15 A long channel while a second anthraniloyl-CoA molecule is waiting in the cleft leading to the protein surface. Cys112, His257, and Asn287 form the FabH-like catalytic triad of PqsD. The C112A mutant is inactive, although it still reversibly binds anthraniloyl-CoA. The covalent complex between anthranilate and Cys112 clearly illuminates the orientation of key elements of the PqsD catalytic machinery and represents a snapshot of a key point in the catalytic cycle.
Figures




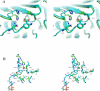



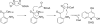

References
-
- Wagner VE, Iglewski BH. P. aeruginosa Biofilms in CF Infection. Clin. Rev. Allergy. Immunol. 2008;35:124–134. - PubMed
-
- Engel J, Balachandran P. Role of Pseudomonas aeruginosa type III effectors in disease. Curr. Opin. Microbiol. 2009;12:61–66. - PubMed
-
- Zelenitsky SA, Harding GK, Sun S, Ubhi K, Ariano RE. Treatment and outcome of Pseudomonas aeruginosa bacteraemia: an antibiotic pharmacodynamic analysis. J. Antimicrob. Chemother. 2003;52:668–674. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

