Histamine-1 receptor is not required as a downstream effector of orexin-2 receptor in maintenance of basal sleep/wake states
- PMID: 19694625
- PMCID: PMC3513392
- DOI: 10.1111/j.1748-1716.2009.02032.x
Histamine-1 receptor is not required as a downstream effector of orexin-2 receptor in maintenance of basal sleep/wake states
Abstract
Aim: The effect of orexin on wakefulness has been suggested to be largely mediated by activation of histaminergic neurones in the tuberomammillary nucleus (TMN) via orexin receptor-2 (OX(2)R). However, orexin receptors in other regions of the brain might also play important roles in maintenance of wakefulness. To dissect the role of the histaminergic system as a downstream mediator of the orexin system in the regulation of sleep/wake states without compensation by the orexin receptor-1 (OX(1)R) mediated pathways, we analysed the phenotype of Histamine-1 receptor (H(1)R) and OX(1)R double-deficient (H(1)R(-/-);OX(1)R(-/-)) mice. These mice lack OX(1)R-mediated pathways in addition to deficiency of H(1)R, which is thought to be the most important system in downstream of OX(2)R.
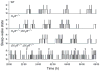
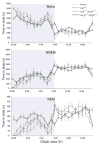
Methods: We used H(1)R deficient (H(1)R(-/-)) mice, H(1)R(-/-);OX(1)R(-/-) mice, OX(1)R and OX(2)R double-deficient (OX(1)R(-/-);OX(2)R(-/-)) mice, and wild type controls. Rapid eye movement (REM) sleep, non-REM (NREM) sleep and awake states were determined by polygraphic electroencephalographic/electromyographic recording.
Results: No abnormality in sleep/wake states was observed in H(1)R(-/-) mice, consistent with previous studies. H(1)R(-/-);OX(1)R(-/-) mice also showed a sleep/wake phenotype comparable to that of wild type mice, while OX(1)R(-/-); OX(2)R(-/-) mice showed severe fragmentation of sleep/wake states.
Conclusion: Our observations showed that regulation of the sleep/wake states is completely achieved by OX(2)R-expressing neurones without involving H(1)R-mediated pathways. The maintenance of basal physiological sleep/wake states is fully achieved without both H(1) and OX(1) receptors. Downstream pathways of OX(2)R other than the histaminergic system might play an important role in the maintenance of sleep/wake states.
Figures

References
-
- Akiyama M, Yuasa T, Hayasaka N, Horikawa K, Sakurai T, Shibata S. Reduced food anticipatory activity in genetically orexin (hypocretin) neuron-ablated mice. Eur J Neurosci. 2004;20:3054–3062. - PubMed
-
- Bouthenet ML, Ruat M, Sales N, Garbarg M, Schwartz JC. A detailed mapping of histamine H1-receptors in guinea-pig central nervous system established by autoradiography with [125I]iodobolpyramine. Neuroscience. 1988;26:553–600. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

