Forward and reverse genetics to identify genes involved in the age-related resistance response in Arabidopsis thaliana
- PMID: 19694953
- PMCID: PMC6640485
- DOI: 10.1111/j.1364-3703.2009.00557.x
Forward and reverse genetics to identify genes involved in the age-related resistance response in Arabidopsis thaliana
Abstract
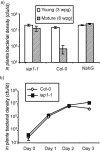
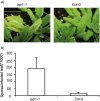
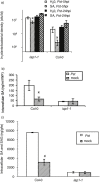
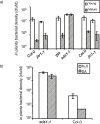
SUMMARY Age-related resistance (ARR) occurs in numerous plant species, often resulting in increased disease resistance as plants mature. ARR in Arabidopsis to Pseudomonas syringae pv. tomato is associated with intercellular salicylic acid (SA) accumulation and the transition to flowering. Forward and reverse genetic screens were performed to identify genes required for ARR and to investigate the mechanism of the ARR response. Infiltration of SA into the intercellular space of the ARR-defective mutant iap1-1 (important for the ARR pathway) partially restored ARR function. Inter- and intracellular SA accumulation was reduced in the mutant iap1-1 compared with the wild-type, and the SA regulatory gene EDS1 was also required for ARR. Combining microarray analysis with reverse genetics using T-DNA insertion lines, four additional ARR genes were identified as contributing to ARR: two plant-specific transcription factors of the NAC family [ANAC055 (At3g15500) and ANAC092 (At5g39610)], a UDP-glucose glucosyltransferase [UGT85A1 (At1g22400)] and a cytidine deaminase [CDA1 (At2g19570)]. These four genes and IAP1 are also required for ARR to Hyaloperonospora parasitica. IAP1 encodes a key component of ARR that acts upstream of SA accumulation and possibly downstream of UGT85A1, CDA1 and the two NAC transcription factors (ANAC055, ANAC092).
Figures


References
-
- Agrios, G.N. (2005. ) Plant Pathology 5th edition London: Elsevier Academic Press.
-
- Aida, M. , Ishida, T. , and Tasaka, M. (1999) Shoot apical meristem and cotyledon formation during Arabidopsis embryogenesis: interaction among the CUP‐SHAPED COTYLEDON and SHOOT MERISTEMLESS genes. Development, 126, 1563–1570. - PubMed
-
- Alonso, J.M. , Stepanova, A.N. , Leisse, T.J. , Kim, C.J. , Chen, H. , Shinn, P. , Stevenson, D.K. , Zimmerman, J. , Barajas, P. , Cheuk, R. , et al (2003) Genome‐wide insertional mutagenesis of Arabidopsis thaliana . Science, 301(5633), 653–657. - PubMed
-
- Brooks, D. , Bender, C. , and Kunkel, BJ. (2005) The Pseudomonas syringae phytotoxin coronatine promotes virulence by overcoming salicylic acid‐dependent defences in Arabidopsis thaliana . Mol. Plant Pathol. 6, 629–639. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

