Akt increases sarcoplasmic reticulum Ca2+ cycling by direct phosphorylation of phospholamban at Thr17
- PMID: 19696029
- PMCID: PMC2788869
- DOI: 10.1074/jbc.M109.036566
Akt increases sarcoplasmic reticulum Ca2+ cycling by direct phosphorylation of phospholamban at Thr17
Abstract
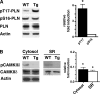
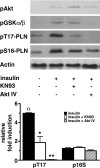
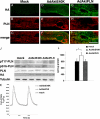
Cardiomyocytes adapt to physical stress by increasing their size while maintaining cell function. The serine/threonine kinase Akt plays a critical role in this process of adaptation. We previously reported that transgenic overexpression of an active form of Akt (Akt-E40K) in mice results in increased cardiac contractility and cell size, as well as improved sarcoplasmic reticulum (SR) Ca(2+) handling. Because it is not fully elucidated, we decided to study the molecular mechanism by which Akt-E40K overexpression improves SR Ca(2+) handling. To this end, SR Ca(2+) uptake and the phosphorylation status of phospholamban (PLN) were evaluated in heart extracts from wild-type and Akt-E40K mice and mice harboring inducible and cardiac specific knock-out of phosphatidylinositol-dependent kinase-1, the upstream activator of Akt. Moreover, the effect of Akt was assessed in vitro by overexpressing a mutant Akt targeted preferentially to the SR, and by biochemical assays to evaluate potential interaction with PLN. We found that when activated, Akt interacts with and phosphorylates PLN at Thr(17), the Ca(2+)-calmodulin-dependent kinase IIdelta site, whereas silencing Akt signaling, through the knock-out of phosphatidylinositol-dependent kinase-1, resulted in reduced phosphorylation of PLN at Thr(17). Furthermore, overexpression of SR-targeted Akt in cardiomyocytes improved Ca(2+) handling without affecting cell size. Thus, we describe here a new mechanism whereby the preferential translocation of Akt to the SR is responsible for enhancement of contractility without stimulation of hypertrophy.
Figures


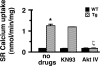
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

