Intestinal cell kinase, a MAP kinase-related kinase, regulates proliferation and G1 cell cycle progression of intestinal epithelial cells
- PMID: 19696144
- PMCID: PMC2763805
- DOI: 10.1152/ajpgi.00066.2009
Intestinal cell kinase, a MAP kinase-related kinase, regulates proliferation and G1 cell cycle progression of intestinal epithelial cells
Abstract
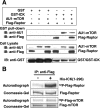
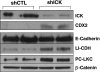
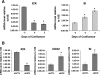
Intestinal cell kinase (ICK), originally cloned from the intestine and expressed in the intestinal crypt epithelium, is a highly conserved serine/threonine protein kinase that is similar to mitogen-activated protein kinases (MAPKs) in the catalytic domain and requires dual phosphorylation within a MAPK-like TDY motif for full activation. Despite these similarities to MAPKs, the biological functions of ICK remain unknown. In this study, we report that suppression of ICK expression in cultured intestinal epithelial cells by short hairpin RNA (shRNA) interference significantly impaired cellular proliferation and induced features of gene expression characteristic of colonic or enterocytic differentiation. Downregulation of ICK altered expression of cell cycle regulators (cyclin D1, c-Myc, and p21(Cip1/WAF1)) of G(1)-S transition, consistent with the G(1) cell cycle delay induced by ICK shRNA. ICK deficiency also led to a significant decrease in the expression and/or activity of p70 ribosomal protein S6 kinase (S6K1) and eukaryotic initiation factor 4E (eIF4E), concomitant with reduced expression of their upstream regulators, the mammalian target of rapamycin (mTOR) and the regulatory associated protein of mTOR (Raptor). Furthermore, ICK interacts with the mTOR/Raptor complex in vivo and phosphorylates Raptor in vitro. These results suggest that disrupting ICK function may downregulate protein translation of specific downstream targets of eIF4E and S6K1 such as cyclin D1 and c-Myc through the mTOR/Raptor signaling pathway. Taken together, our findings demonstrate an important role for ICK in proliferation and differentiation of intestinal epithelial cells.
Figures




References
-
- Abe S, Yagi T, Ishiyama S, Hiroe M, Marumo F, Ikawa Y. Molecular cloning of a novel serine/threonine kinase, MRK, possibly involved in cardiac development. Oncogene 11: 2187–2195, 1995 - PubMed
-
- Aliaga JC, Deschenes C, Beaulieu JF, Calvo EL, Rivard N. Requirement of the MAP kinase cascade for cell cycle progression and differentiation of human intestinal cells. Am J Physiol Gastrointest Liver Physiol 277: G631–G641, 1999 - PubMed
-
- Aoki K, Tamai Y, Horiike S, Oshima M, Taketo MM. Colonic polyposis caused by mTOR-mediated chromosomal instability in Apc+/ Delta716 Cdx2+/− compound mutant mice. Nat Genet 35: 323–330, 2003 - PubMed
-
- Beachy PA, Karhadkar SS, Berman DM. Tissue repair and stem cell renewal in carcinogenesis. Nature 432: 324–331, 2004 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

