Caveolin-1 and lipid microdomains regulate Gs trafficking and attenuate Gs/adenylyl cyclase signaling
- PMID: 19696145
- PMCID: PMC2774991
- DOI: 10.1124/mol.109.060160
Caveolin-1 and lipid microdomains regulate Gs trafficking and attenuate Gs/adenylyl cyclase signaling
Expression of concern in
-
Expression of concern: "Caveolin-1 and Lipid Microdomains Regulate Gs Trafficking and Attenuate Gs/Adenylyl Cyclase Signaling" [Molecular Pharmacology, Volume 76, Issue 5, November 2009, Pages 1082-1093].Mol Pharmacol. 2025 Mar;107(3):100023. doi: 10.1016/j.molpha.2025.100023. Epub 2025 Feb 20. Mol Pharmacol. 2025. PMID: 40157804 Free PMC article. No abstract available.
Abstract
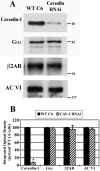
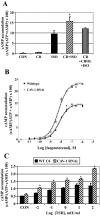
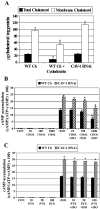
Lipid rafts and caveolae are specialized membrane microdomains implicated in regulating G protein-coupled receptor signaling cascades. Previous studies have suggested that rafts/caveolae may regulate beta-adrenergic receptor/Galpha(s) signaling, but underlying molecular mechanisms are largely undefined. Using a simplified model system in C6 glioma cells, this study disrupts rafts/caveolae using both pharmacological and genetic approaches to test whether caveolin-1 and lipid microdomains regulate G(s) trafficking and signaling. Lipid rafts/caveolae were disrupted in C6 cells by either short-term cholesterol chelation using methyl-beta-cyclodextrin or by stable knockdown of caveolin-1 and -2 by RNA interference. In imaging studies examining Galpha(s)-GFP during signaling, stimulation with the betaAR agonist isoproterenol resulted in internalization of Galpha(s)-GFP; however, this trafficking was blocked by methyl-beta-cyclodextrin or by caveolin knockdown. Caveolin knockdown significantly decreased Galpha(s) localization in detergent insoluble lipid raft/caveolae membrane fractions, suggesting that caveolin localizes a portion of Galpha(s) to these membrane microdomains. Methyl-beta-cyclodextrin or caveolin knockdown significantly increased isoproterenol or thyrotropin-stimulated cAMP accumulation. Furthermore, forskolin- and aluminum tetrafluoride-stimulated adenylyl cyclase activity was significantly increased by caveolin knockdown in cells or in brain membranes obtained from caveolin-1 knockout mice, indicating that caveolin attenuates signaling at the level of Galpha(s)/adenylyl cyclase and distal to GPCRs. Taken together, these results demonstrate that caveolin-1 and lipid microdomains exert a major effect on Galpha(s) trafficking and signaling. It is suggested that lipid rafts/caveolae are sites that remove Galpha(s) from membrane signaling cascades and caveolins might dampen globally Galpha(s)/adenylyl cyclase/cAMP signaling.
Figures




References
-
- Allen JA, Halverson-Tamboli RA, Rasenick MM. (2007) Lipid raft microdomains and neurotransmitter signalling. Nat Rev Neurosci 8:128–140 - PubMed
-
- Allen JA, Yu JZ, Donati RJ, Rasenick MM. (2005) β-Adrenergic receptor stimulation promotes Gαs internalization through lipid rafts: a study in living cells. Mol Pharmacol 67:1493–1504 - PubMed
-
- Bhatnagar A, Sheffler DJ, Kroeze WK, Compton-Toth B, Roth BL. (2004) Caveolin-1 interacts with 5-HT2A serotonin receptors and profoundly modulates the signaling of selected Gαq-coupled protein receptors. J Biol Chem 279:34614–34623 - PubMed
-
- Bradford MM. (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254 - PubMed
-
- Brown DA. (2006) Lipid rafts, detergent-resistant membranes, and raft targeting signals. Physiology (Bethesda) 21:430–439 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 DA027170/DA/NIDA NIH HHS/United States
- R21-DA20568/DA/NIDA NIH HHS/United States
- T32-HD040127/HD/NICHD NIH HHS/United States
- T32 HD040127/HD/NICHD NIH HHS/United States
- T32-HL07692/HL/NHLBI NIH HHS/United States
- T32 HL007692/HL/NHLBI NIH HHS/United States
- R01 MH078200/MH/NIMH NIH HHS/United States
- R21 DA020568/DA/NIDA NIH HHS/United States
- R01 MH039595/MH/NIMH NIH HHS/United States
- R01-MH39595/MH/NIMH NIH HHS/United States
- R01 MH061887/MH/NIMH NIH HHS/United States
- R01-MH78200/MH/NIMH NIH HHS/United States
- R01-MH61887/MH/NIMH NIH HHS/United States
LinkOut - more resources
Full Text Sources

