Dysfunctional expansion of hematopoietic stem cells and block of myeloid differentiation in lethal sepsis
- PMID: 19696201
- PMCID: PMC2774548
- DOI: 10.1182/blood-2009-04-214916
Dysfunctional expansion of hematopoietic stem cells and block of myeloid differentiation in lethal sepsis
Abstract
Severe sepsis is one of the leading causes of death worldwide. High mortality rates in sepsis are frequently associated with neutropenia. Despite the central role of neutrophils in innate immunity, the mechanisms causing neutropenia during sepsis remain elusive. Here, we show that neutropenia is caused in part by apoptosis and is sustained by a block of hematopoietic stem cell (HSC) differentiation. Using a sepsis murine model, we found that the human opportunistic bacterial pathogen Pseudomonas aeruginosa caused neutrophil depletion and expansion of the HSC pool in the bone marrow. "Septic" HSCs were significantly impaired in competitive repopulation assays and defective in generating common myeloid progenitors and granulocyte-monocyte progenitors, resulting in lower rates of myeloid differentiation in vitro and in vivo. Delayed myeloid-neutrophil differentiation was further mapped using a lysozyme-green fluorescent protein (GFP) reporter mouse. Pseudomonas's lipopolysaccharide was necessary and sufficient to induce myelosuppresion and required intact TLR4 signaling. Our results establish a previously unrecognized link between HSC regulation and host response in severe sepsis and demonstrate a novel role for TLR4.
Figures
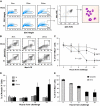
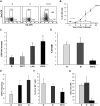
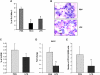
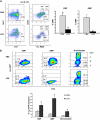
 ) and long-term HSCs (LT-HSCs; ■) on total Lin− population. Values are average of 12 mice and summarize 3 independent experiments. Error bars indicate SE. *P < .001. (D) In each experiment, a sample of sorted LSK was derived from a pool of BM of 4 to 6 mice. RNA was extracted from LSK (IL-7R−) cells from PBS- and LPS-challenged mice. Samples were analyzed for expression of C/EBPα and PU.1 by qRT-PCR. Bar graphs represent averages of fold changes in expression in 3 independent samples. Error bars indicate SD. LPS versus PBS: C/EBPα, *P = .033; PU.1 *P = .029. (E) Sepsis causes alterations in HSC differentiation (working model). During severe sepsis, bacterial LPS induces TLR4-dependent expansion of dysfunctional LSK cells displaying a defective ability to progress into the pool of myeloid progenitors: CMPs and GMPs. Reduction in CMPs and GMPs results in neutropenia.
) and long-term HSCs (LT-HSCs; ■) on total Lin− population. Values are average of 12 mice and summarize 3 independent experiments. Error bars indicate SE. *P < .001. (D) In each experiment, a sample of sorted LSK was derived from a pool of BM of 4 to 6 mice. RNA was extracted from LSK (IL-7R−) cells from PBS- and LPS-challenged mice. Samples were analyzed for expression of C/EBPα and PU.1 by qRT-PCR. Bar graphs represent averages of fold changes in expression in 3 independent samples. Error bars indicate SD. LPS versus PBS: C/EBPα, *P = .033; PU.1 *P = .029. (E) Sepsis causes alterations in HSC differentiation (working model). During severe sepsis, bacterial LPS induces TLR4-dependent expansion of dysfunctional LSK cells displaying a defective ability to progress into the pool of myeloid progenitors: CMPs and GMPs. Reduction in CMPs and GMPs results in neutropenia.
 ) and long-term HSCs (LT-HSCs; ■) on total Lin− population. Values are average of 12 mice and summarize 3 independent experiments. Error bars indicate SE. *P < .001. (D) In each experiment, a sample of sorted LSK was derived from a pool of BM of 4 to 6 mice. RNA was extracted from LSK (IL-7R−) cells from PBS- and LPS-challenged mice. Samples were analyzed for expression of C/EBPα and PU.1 by qRT-PCR. Bar graphs represent averages of fold changes in expression in 3 independent samples. Error bars indicate SD. LPS versus PBS: C/EBPα, *P = .033; PU.1 *P = .029. (E) Sepsis causes alterations in HSC differentiation (working model). During severe sepsis, bacterial LPS induces TLR4-dependent expansion of dysfunctional LSK cells displaying a defective ability to progress into the pool of myeloid progenitors: CMPs and GMPs. Reduction in CMPs and GMPs results in neutropenia.
) and long-term HSCs (LT-HSCs; ■) on total Lin− population. Values are average of 12 mice and summarize 3 independent experiments. Error bars indicate SE. *P < .001. (D) In each experiment, a sample of sorted LSK was derived from a pool of BM of 4 to 6 mice. RNA was extracted from LSK (IL-7R−) cells from PBS- and LPS-challenged mice. Samples were analyzed for expression of C/EBPα and PU.1 by qRT-PCR. Bar graphs represent averages of fold changes in expression in 3 independent samples. Error bars indicate SD. LPS versus PBS: C/EBPα, *P = .033; PU.1 *P = .029. (E) Sepsis causes alterations in HSC differentiation (working model). During severe sepsis, bacterial LPS induces TLR4-dependent expansion of dysfunctional LSK cells displaying a defective ability to progress into the pool of myeloid progenitors: CMPs and GMPs. Reduction in CMPs and GMPs results in neutropenia.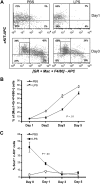


References
-
- Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. N Engl J Med. 2003;348(2):138–150. - PubMed
-
- Nathan C. Neutrophils and immunity: challenges and opportunities. Nat Rev Immunol. 2006;6(3):173–182. - PubMed
-
- Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol. 2007;7(9):678–689. - PubMed
-
- Blank U, Karlsson G, Karlsson S. Signaling pathways governing stem-cell fate. Blood. 2008;111(2):492–503. - PubMed
-
- Scadden DT. The stem-cell niche as an entity of action. Nature. 2006;441(7097):1075–1079. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

