Technical breakthroughs in the wearable artificial kidney (WAK)
- PMID: 19696219
- PMCID: PMC2736696
- DOI: 10.2215/CJN.02790409
Technical breakthroughs in the wearable artificial kidney (WAK)
Abstract
Background: The wearable artificial kidney (WAK) has been a holy grail in kidney failure for decades. Described herein are the breakthroughs that made possible the creation of the WAK V1.0 and its advanced versions V 1.1 and 1.2.
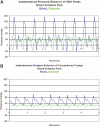
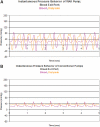
Design: The battery-powered WAK pump has a double channel pulsatile counter phase flow. This study clarifies the role of pulsatile blood and dialysate flow, a high-flux membrane with a larger surface area, and the optimization of the dialysate pH. Flows and clearances from the WAK pump were compared with conventional pumps and with gravity steady flow.
Results: Raising dialysate pH to 7.4 increased adsorption of ammonia. Clearances were higher with pulsatile flow as compared with steady flow. The light WAK pump, geometrically suitable for wearability, delivered the same clearances as larger and heavier pumps that cannot be battery operated. Beta(2) microglobulin (beta(2)M) was removed from human blood in vitro. Activated charcoal adsorbed most beta(2)M in the dialysate. The WAK V1.0 delivered an effective creatinine clearance of 18.5 +/- 3.2 ml/min and the WAK V1.1 27.0 +/- 4.0 ml/min in uremic pigs.
Conclusions: Half-cycle differences between blood and dialysate, alternating transmembrane pressures (TMP), higher amplitude pulsations, and a push-pull flow increased convective transport. This creates a yet undescribed type of hemodiafiltration. Further improvements were achieved with a larger surface area high-flux dialyzer and a higher dialysate pH. The data suggest that the WAK might be an efficient way of providing daily dialysis and optimizing end stage renal disease (ESRD) treatment.
Figures





Comment in
-
Will nephrologists use a wearable artificial kidney?Clin J Am Soc Nephrol. 2009 Sep;4(9):1401-2. doi: 10.2215/CJN.04600709. Epub 2009 Aug 6. Clin J Am Soc Nephrol. 2009. PMID: 19661217 No abstract available.
References
-
- Lockridge RS: The direction of end-stage renal disease reimbursement in the United States. Semin Dial 17: 125–130, 2004 - PubMed
-
- Lockridge RS, McKinney JK: Is HCFA's reimbursement policy controlling quality of care for end-stage renal disease patients? ASAIO J 47: 466–468, 2001 - PubMed
-
- Manns BJ, Johnson JA, Taub K, Mortis G, Ghali WA, Donaldson C: Dialysis adequacy and health related quality of life in hemodialysis patients. ASAIO J 48: 565–569, 2002 - PubMed
-
- Mapes DL, Lopes AA, Satayathum S, McCullough KP, Goodkin DA, Locatelli F, Fukuhara S, Young EW, Kurokawa K, Saito A, Bommer J, Wolfe RA, Held PJ, Port FK: Health-related quality of life as a predictor of mortality and hospitalization: The Dialysis Outcomes and Practice Patterns Study (DOPPS). Kidney Int 64: 339–349, 2003 - PubMed
-
- McFarlane PA, Bayoumi AM, Pierratos A, Redelmeier DA: The quality of life and cost utility of home nocturnal and conventional in-center hemodialysis. Kidney Int 64: 1004–1011, 2003 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

