Scavenger receptors of endothelial cells mediate the uptake and cellular proatherogenic effects of carbamylated LDL
- PMID: 19696406
- PMCID: PMC5075391
- DOI: 10.1161/ATVBAHA.109.189795
Scavenger receptors of endothelial cells mediate the uptake and cellular proatherogenic effects of carbamylated LDL
Abstract
Objective: Carbamylated LDL (cLDL) has been recently shown to have robust proatherogenic effects on human endothelial cells in vitro, suggesting cLDL may have a significant role in atherosclerosis in uremia. The current study was designed to determine which receptors are used by cLDL and thus cause the proatherogenic effects.
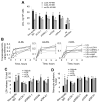
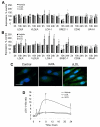
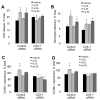
Methods and results: In ex vivo or in vitro models as well as in intact animals, administration of cLDL was associated with endothelial internalization of cLDL and subendothelial translocation (transcytosis). In vitro recombinant LOX-1 and SREC-1 receptors showed the greatest cLDL binding. However, pretreatment of the endothelial cells with specific inhibiting antibodies demonstrated that cLDL binds mainly to LOX-1 and CD36 receptors. The transcytosis was dependent on SR-A1, SREC-1, and CD36 receptors whereas LOX-1 receptor was not involved. The cytotoxicity was mediated by several studied scavenger receptors, but cLDL-induced monocyte adhesion depended only on LOX-1. The cLDL-induced synthesis of LOX-1 protein significantly contributed to both cytotoxicity and accelerated monocyte adhesion to endothelial cells.
Conclusions: Our data suggest that cLDL uses a unique pattern of scavenger receptors. They show that LOX-1 receptor, and partially CD36, SREC-1, and SR-A1 receptors, are essential for the proatherogenic effects of cLDL on human endothelial cells.
Figures


References
-
- Kraus LM, Kraus AP., Jr. Carbamoylation of amino acids and proteins in uremia. Kidney Int Suppl. 2001;78:S102–107. - PubMed
-
- Apostolov EO, Shah SV, Ok E, Basnakian AG. Quantification of carbamylated LDL in human sera by a new sandwich ELISA. Clin Chem. 2005;51:719–728. - PubMed
-
- Wang Z, Nicholls SJ, Rodriguez ER, Kummu O, Horkko S, Barnard J, Reynolds WF, Topol EJ, DiDonato JA, Hazen SL. Protein carbamylation links inflammation, smoking, uremia and atherogenesis. Nat Med. 2007;13:1176–1184. - PubMed
-
- Apostolov EO, Basnakian AG, Yin X, Ok E, Shah SV. Modified LDLs induce proliferation-mediated death of human vascular endothelial cells through MAPK pathway. Am J Physiol Heart Circ Physiol. 2007;292:H1836–1846. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

