Successful reduction of immunosuppression in older renal transplant recipients who exhibit donor-specific regulation
- PMID: 19696637
- PMCID: PMC2747510
- DOI: 10.1097/TP.0b013e3181b0f92f
Successful reduction of immunosuppression in older renal transplant recipients who exhibit donor-specific regulation
Abstract
Background: We hypothesized that T-regulatory cells specific for donor alloantigens would protect a renal transplant during partial withdrawal of immunosuppression.
Methods: To test this hypothesis, 32 renal transplant recipients aged 55 years and older with excellent renal function were tested for donor-specific regulation (DSR) by trans-vivo delayed type hypersensitivity assay at the time of enrollment (T=0) and 6 months later (T=6). Twenty-two patients had prednisone withdrawn during a 3-month period, whereas 10 controls were maintained on triple therapy (prednisone, cyclosporine, and mycophenolate).
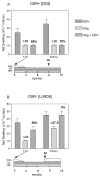
Results: Of 22 patients in the steroid withdrawal group, 10 were DSR+ and 12 were DSR- at the time of enrollment (T=0). None of the DSR+ patients experienced acute rejection, nor did any have donor-specific human leukocyte antigen (HLA) antibody during or after withdrawal. Of 12 DSR- patients, three developed acute rejection, which were reversed with bolus steroid treatment, and four were donor-specific antibody+ at T=0 or T=6. Two years later, 80% (8 of 10) of DSR+ patients in the withdrawal group remain steroid free while maintaining excellent renal function, as compared with only 58% (7 of 12) DSR- patients. Patient survival at 4 years was similar for DSR+ (9 of 10) and DSR- (11 of 12) patients in the withdrawal group. Patients maintained on triple therapy remained rejection free during the 4-year follow-up regardless of initial DSR status, with patient survival rate of 70% (7 of 10).
Conclusions: DSR before steroid withdrawal may identify a subset of transplant patients who could benefit from reduction of immunosuppression without elevated risk of rejection or deteriorating renal function.
Figures


References
-
- Nankivell BJ, Borrows RJ, Fung CL, O’Connell PJ, Allen RD, Chapman JR. The natural history of chronic allograft nephropathy. N Engl J Med. 2003;349 (24):2326. - PubMed
-
- Lechler RI, Sykes M, Thomson AW, Turka LA. Organ transplantation--how much of the promise has been realized? Nat Med. 2005;11 (6):605. - PubMed
-
- Augustine JJ, Hricik DE. Minimization of immunosuppression in kidney transplantation. Curr Opin Nephrol Hypertens. 2007;16 (6):535. - PubMed
-
- Keunecke C, Rothenpieler U, Zanker B, et al. Mycophenolate mofetil monotherapy: an example of a safe nephrotoxicity/atherogenicity-free immunosuppressive maintenance regimen in a selected group of kidney-transplanted patients. Transplant Proc. 2000 Feb;32(1A Suppl):6S. - PubMed
-
- Veenstra DL, Best JH, Hornberger J, Sullivan SD, Hricik DE. Incidence and long-term cost of steroid-related side effects after renal transplantation. Am J Kidney Dis. 1999;33 (5):829. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

