Regulated activity of PP2A-B55 delta is crucial for controlling entry into and exit from mitosis in Xenopus egg extracts
- PMID: 19696736
- PMCID: PMC2750019
- DOI: 10.1038/emboj.2009.238
Regulated activity of PP2A-B55 delta is crucial for controlling entry into and exit from mitosis in Xenopus egg extracts
Abstract
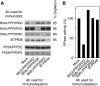
Entry into mitosis depends on the activity of cyclin-dependent kinases (CDKs). Conversely, exit from mitosis occurs when mitotic cyclins are degraded, thereby extinguishing CDK activity. Exit from mitosis must also require mitotic phosphoproteins to revert to their interphase hypophosphorylated forms, but there is a controversy about which phosphatase(s) is/are responsible for dephosphorylating the CDK substrates. We find that PP2A associated with a B55 delta subunit is relatively specific for a model mitotic CDK substrate in Xenopus egg extracts. The phosphatase activity measured by this substrate is regulated during the cell cycle--high in interphase and suppressed during mitosis. Depletion of PP2A-B55 delta (in interphase) from 'cycling' frog egg extracts accelerated their entry into mitosis and kept them indefinitely in mitosis. When PP2A-B55 delta was depleted from mitotic extracts, however, exit from mitosis was hardly delayed, showing that other phosphatase(s) are also required for mitotic exit. Increasing the concentration of PP2A-B55 delta in extracts by adding recombinant enzyme inhibited the entry into mitosis. This form of PP2A seems to be a key regulator of entry into and exit from mitosis.
Figures

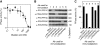


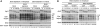
References
-
- Berdougo E, Nachury MV, Jackson PK, Jallepalli PV (2008) The nucleolar phosphatase Cdc14B is dispensable for chromosome segregation and mitotic exit in human cells. Cell Cycle 7: 1184–1190 - PubMed
-
- Chen F, Archambault V, Kar A, Lio P, D'Avino PP, Sinka R, Lilley K, Laue ED, Deak P, Capalbo L, Glover DM (2007) Multiple protein phosphatases are required for mitosis in Drosophila. Curr Biol 17: 293–303 - PubMed
-
- Doonan JH, Morris NR (1989) The bimG gene of Aspergillus nidulans, required for completion of anaphase, encodes a homolog of mammalian phosphoprotein phosphatase 1. Cell 57: 987–996 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

