Rho family GTPases and their regulators in lymphocytes
- PMID: 19696767
- PMCID: PMC4898593
- DOI: 10.1038/nri2606
Rho family GTPases and their regulators in lymphocytes
Abstract
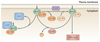
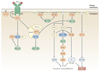
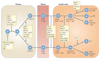
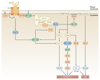
Rho family GTPases, and the proteins that regulate them, have important roles in many cellular processes, including cell division, survival, migration and adhesion. Although most of our understanding of these proteins has come from studies using cell lines, more recent gene targeting studies in mice are providing insights into the in vivo function of these proteins. Here we review recent progress revealing crucial roles for these proteins in lymphocyte development, activation, differentiation and migration. The emerging picture shows that Rho family GTPases transduce signals from receptors for antigens, chemokines and cytokines, as well as adhesion molecules and pattern recognition receptors, and that they function as focal points for crosstalk between different signalling pathways.
Figures



References
-
- Heasman SJ, Ridley AJ. Mammalian Rho GTPases: new insights into their functions from in vivo studies. Nat Rev Mol Cell Biol. 2008;9:690–701. - PubMed
-
- Wennerberg K, Der CJ. Rho-family GTPases: it's not only Rac and Rho (and I like it) J Cell Sci. 2004;117:1301–1312. - PubMed
-
- Rossman KL, Der CJ, Sondek J. GEF means go: turning on RHO GTPases with guanine nucleotide-exchange factors. Nat Rev Mol Cell Biol. 2005;6:167–180. - PubMed
-
- Tcherkezian J, Lamarche-Vane N. Current knowledge of the large RhoGAP family of proteins. Biol Cell. 2007;99:67–86. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

