Pathways and mechanisms of endocytic recycling
- PMID: 19696797
- PMCID: PMC3038567
- DOI: 10.1038/nrm2755
Pathways and mechanisms of endocytic recycling
Abstract
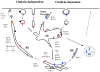
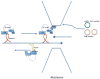
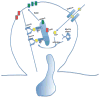
Endocytic recycling is coordinated with endocytic uptake to control the composition of the plasma membrane. Although much of our understanding of endocytic recycling has come from studies on the transferrin receptor, a protein internalized through clathrin-dependent endocytosis, increased interest in clathrin-independent endocytosis has led to the discovery of new endocytic recycling systems. Recent insights into the regulatory mechanisms that control endocytic recycling have focused on recycling through tubular carriers and the return to the cell surface of cargoes that enter cells through clathrin-independent mechanisms. Recent work emphasizes the importance of regulated recycling in processes as diverse as cytokinesis, cell adhesion, morphogenesis, cell fusion, learning and memory.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

