Expression of stem cell markers in the human fetal kidney
- PMID: 19696931
- PMCID: PMC2725321
- DOI: 10.1371/journal.pone.0006709
Expression of stem cell markers in the human fetal kidney
Erratum in
- PLoS One. 2011;6(3). doi: 10.1371/annotation/5c42ca40-0a01-4e4e-94e3-ccaa2cc54fc6 doi: 10.1371/annotation/5c42ca40-0a01-4e4e-94e3-ccaa2cc54fc6
Abstract
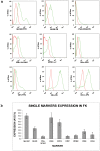
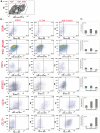
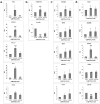
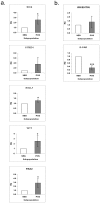
In the human fetal kidney (HFK) self-renewing stem cells residing in the metanephric mesenchyme (MM)/blastema are induced to form all cell types of the nephron till 34(th) week of gestation. Definition of useful markers is crucial for the identification of HFK stem cells. Because wilms' tumor, a pediatric renal cancer, initiates from retention of renal stem cells, we hypothesized that surface antigens previously up-regulated in microarrays of both HFK and blastema-enriched stem-like wilms' tumor xenografts (NCAM, ACVRIIB, DLK1/PREF, GPR39, FZD7, FZD2, NTRK2) are likely to be relevant markers. Comprehensive profiling of these putative and of additional stem cell markers (CD34, CD133, c-Kit, CD90, CD105, CD24) in mid-gestation HFK was performed using immunostaining and FACS in conjunction with EpCAM, an epithelial surface marker that is absent from the MM and increases along nephron differentiation and hence can be separated into negative, dim or bright fractions. No marker was specifically localized to the MM. Nevertheless, FZD7 and NTRK2 were preferentially localized to the MM and emerging tubules (<10% of HFK cells) and were mostly present within the EpCAM(neg) and EpCAM(dim) fractions, indicating putative stem/progenitor markers. In contrast, single markers such as CD24 and CD133 as well as double-positive CD24(+)CD133(+) cells comprise >50% of HFK cells and predominantly co-express EpCAM(bright), indicating they are mostly markers of differentiation. Furthermore, localization of NCAM exclusively in the MM and in its nephron progenitor derivatives but also in stroma and the expression pattern of significantly elevated renal stem/progenitor genes Six2, Wt1, Cited1, and Sall1 in NCAM(+)EpCAM(-) and to a lesser extent in NCAM(+)EpCAM(+) fractions confirmed regional identity of cells and assisted us in pinpointing the presence of subpopulations that are putative MM-derived progenitor cells (NCAM(+)EpCAM(+)FZD7(+)), MM stem cells (NCAM(+)EpCAM(-)FZD7(+)) or both (NCAM(+)FZD7(+)). These results and concepts provide a framework for developing cell selection strategies for human renal cell-based therapies.
Conflict of interest statement
Figures




References
-
- Brodbeck S, Englert C. Genetic determination of nephrogenesis: the Pax/Eya/Six gene network. Pediatr Nephrol. 2004;19(3):249–255. - PubMed
-
- Challen GA, Martinez G, Davis MJ, Taylor DF, Crowe M, et al. Identifying the molecular phenotype of renal progenitor cells. J Am Soc Nephrol. 2004;15(9):2344–2357. - PubMed
-
- Cho EA, Dressler GR. San Diego: San Diego: Academic Press; 2003. In The Kidney: From Normal Development to Congenital Disease. pp. 195–210.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

