Challenges in cardiac tissue engineering
- PMID: 19698068
- PMCID: PMC2946883
- DOI: 10.1089/ten.TEB.2009.0352
Challenges in cardiac tissue engineering
Abstract
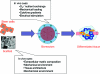
Cardiac tissue engineering aims to create functional tissue constructs that can reestablish the structure and function of injured myocardium. Engineered constructs can also serve as high-fidelity models for studies of cardiac development and disease. In a general case, the biological potential of the cell-the actual "tissue engineer"-is mobilized by providing highly controllable three-dimensional environments that can mediate cell differentiation and functional assembly. For cardiac regeneration, some of the key requirements that need to be met are the selection of a human cell source, establishment of cardiac tissue matrix, electromechanical cell coupling, robust and stable contractile function, and functional vascularization. We review here the potential and challenges of cardiac tissue engineering for developing therapies that could prevent or reverse heart failure.
Figures



References
-
- Harrison R.G. Observations on the living developing nerve fiber. Proc Soc Exp Biol Med. 1907;4:140.
-
- Langer R. Vacanti J.P. Tissue Eng Sci. 1993;260:920. - PubMed
-
- Lanza R. Langer R. Vacanti J. Principles of Tissue Engineering. Boston: Elsevier Academic Press; 2007.
-
- Severs N.J. The cardiac muscle cell. Bioessays. 2000;22:188. - PubMed
-
- American Heart Association Cardiovascular Disease Statistics. 2008.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

