Efficacy of artesunate-amodiaquine for treating uncomplicated falciparum malaria in sub-Saharan Africa: a multi-centre analysis
- PMID: 19698172
- PMCID: PMC2745424
- DOI: 10.1186/1475-2875-8-203
Efficacy of artesunate-amodiaquine for treating uncomplicated falciparum malaria in sub-Saharan Africa: a multi-centre analysis
Abstract
Background: Artesunate and amodiaquine (AS&AQ) is at present the world's second most widely used artemisinin-based combination therapy (ACT). It was necessary to evaluate the efficacy of ACT, recently adopted by the World Health Organization (WHO) and deployed over 80 countries, in order to make an evidence-based drug policy.
Methods: An individual patient data (IPD) analysis was conducted on efficacy outcomes in 26 clinical studies in sub-Saharan Africa using the WHO protocol with similar primary and secondary endpoints.
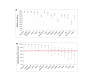
Results: A total of 11,700 patients (75% under 5 years old), from 33 different sites in 16 countries were followed for 28 days. Loss to follow-up was 4.9% (575/11,700). AS&AQ was given to 5,897 patients. Of these, 82% (4,826/5,897) were included in randomized comparative trials with polymerase chain reaction (PCR) genotyping results and compared to 5,413 patients (half receiving an ACT). AS&AQ and other ACT comparators resulted in rapid clearance of fever and parasitaemia, superior to non-ACT. Using survival analysis on a modified intent-to-treat population, the Day 28 PCR-adjusted efficacy of AS&AQ was greater than 90% (the WHO cut-off) in 11/16 countries. In randomized comparative trials (n = 22), the crude efficacy of AS&AQ was 75.9% (95% CI 74.6-77.1) and the PCR-adjusted efficacy was 93.9% (95% CI 93.2-94.5). The risk (weighted by site) of failure PCR-adjusted of AS&AQ was significantly inferior to non-ACT, superior to dihydroartemisinin-piperaquine (DP, in one Ugandan site), and not different from AS+SP or AL (artemether-lumefantrine). The risk of gametocyte appearance and the carriage rate of AS&AQ was only greater in one Ugandan site compared to AL and DP, and lower compared to non-ACT (p = 0.001, for all comparisons). Anaemia recovery was not different than comparator groups, except in one site in Rwanda where the patients in the DP group had a slower recovery.
Conclusion: AS&AQ compares well to other treatments and meets the WHO efficacy criteria for use against falciparum malaria in many, but not all, the sub-Saharan African countries where it was studied. Efficacy varies between and within countries. An IPD analysis can inform general and local treatment policies. Ongoing monitoring evaluation is required.
Figures
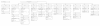



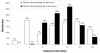


References
-
- World Health Organization Global anti-malarial drug policy database, Africa http://www.who.int/malaria/amdp/amdp_afro.htm
-
- World Health Organization Guidelines for the treatment of malaria. Geneva. 2006. http://www.who.int/malaria/docs/TreatmentGuidelines2006.pdf
-
- Djimdé AA, Fofana B, Sagara I, Sidibe B, Toure S, Dembele D, Dama S, Ouologuem D, Dicko A, Doumbo OK. Efficacy, safety, and selection of molecular markers of drug resistance by two ACTs in Mali. Am J Trop Med Hyg. 2008;78:455–461. - PubMed
-
- Ndiaye JL, Randrianarivelojosia M, Sagara I, Brasseur P, Ndiaye I, Faye B, Randrianasolo L, Ratsimbasoa A, Forlemu D, Moor VA, Traore A, Niawanlou Dara YD, Lameyre V, Diallo M, Djimde A, Same-Ekobo A, Gaye O. Randomized, multicentre assessment of the efficacy and safety of ASAQ – a fixed-dose artesunate-amodiaquine combination therapy in the treatment of uncomplicated Plasmodium falciparum malaria. Malar J. 2009;8:125. doi: 10.1186/1475-2875-8-125. - DOI - PMC - PubMed
-
- Sirima SB, Tiono AB, Gansane A, Diarra A, Ouedraogo A, Konate AT, Kiechel JR, Morgan CC, Olliaro PL, Taylor WR. The efficacy and safety of a new fixed-dose combination of amodiaquine and artesunate in young African children with acute uncomplicated Plasmodium falciparum. Malar J. 2009;8:48. doi: 10.1186/1475-2875-8-48. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

