Aging, age-related macular degeneration, and the response-to-retention of apolipoprotein B-containing lipoproteins
- PMID: 19698799
- PMCID: PMC4319375
- DOI: 10.1016/j.preteyeres.2009.08.001
Aging, age-related macular degeneration, and the response-to-retention of apolipoprotein B-containing lipoproteins
Abstract
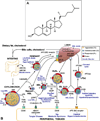
The largest risk factor for age-related macular degeneration (ARMD) is advanced age. A prominent age-related change in the human retina is the accumulation of histochemically detectable neutral lipid in normal Bruch's membrane (BrM) throughout adulthood. This change has the potential to have a major impact on physiology of the retinal pigment epithelium (RPE). It occurs in the same compartment as drusen and basal linear deposit, the pathognomonic extracellular, lipid-containing lesions of ARMD. Here we present evidence from light microscopic histochemistry, ultrastructure, lipid profiling of tissues and isolated lipoproteins, and gene expression analysis that this deposition can be accounted for by esterified cholesterol-rich, apolipoprotein B-containing lipoprotein particles constitutively produced by the RPE. This work collectively allows ARMD lesion formation and its aftermath to be conceptualized as a response to the retention of a sub-endothelial apolipoprotein B lipoprotein, similar to a widely accepted model of atherosclerotic coronary artery disease (CAD) (Tabas et al., 2007). This approach provides a wide knowledge base and sophisticated clinical armamentarium that can be readily exploited for the development of new model systems and the future benefit of ARMD patients.
Figures











References
-
- Executive summary of the third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. - PubMed
-
- Adams CWM, Bayliss OB. Lipid histochemistry. In: Glick D, Rosenbaum RM, editors. Techniques of Biochemical and Biophysical Morphology. New York: John Wiley and Sons; 1975. pp. 100–156.
-
- Adiels M, Olofsson SO, Taskinen MR, Borén J. Overproduction of very low-density lipoproteins is the hallmark of the dyslipidemia in the metabolic syndrome. Arterioscler. Thromb. Vasc. Biol. 2008;28:1225–1236. - PubMed
-
- Afshari FT, Fawcett JW. Improving RPE adhesion to Bruch’s membrane. Eye. 2009 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

