The utility of screening in the design of trials for symptom management in cancer
- PMID: 19699052
- PMCID: PMC2761530
- DOI: 10.1016/j.jpainsymman.2009.02.233
The utility of screening in the design of trials for symptom management in cancer
Abstract
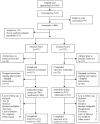
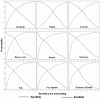
Clinical trials that test interventions for symptom management must target patients whose symptoms are severe and can benefit from participation. Screening symptoms for their severity prior to trial entry may be an important element of trial design. This research describes the utility of screening for severity of symptoms prior to entry into clinical trials for symptom management in cancer. To accomplish this, 601 cancer patients undergoing chemotherapy were assessed at screening and at the initial intervention contact, using the 0-10 rating scale for severity of nine symptoms. Post-test probabilities and likelihood ratios (LRs) were estimated across cut-offs in screening severity scores. Areas under receiver operating characteristic curves for reaching threshold of four at the initial intervention contact were estimated by a nonparametric method. It was found that screening severity scores were good predictors for identifying patients who would not reach threshold but did not always accurately predict patients who would. The cut-offs between 2 and 4 on a 0-10 scale could be used to identify patients that might benefit from receipt of interventions. For all symptoms, the LRs were greater than one across possible screening cut-offs. The findings indicate that decision rules based on screening prior to entry into cancer symptom management trials can provide reasonable discriminative accuracy by differentiating among patients who are likely to reach higher levels of severity later in the trial from those who are not. Optimal severity cut-offs can be established based on LRs and desired sensitivity and specificity.
Figures
References
-
- National Comprehensive Cancer Network Clinical practice guidelines in oncology: cancer-related fatigue. 2006. Available from: http://www.nccn.org/professionals/physician_gls/PDF/fatigue.pdf. Accessed June 28, 2006. - PubMed
-
- Kroneke K. Studying symptoms: sampling and measurement issues. Ann Intern Med. 2001;134:844–853. - PubMed
-
- Komaroff AL. Symptoms: in the head or in the brain? Ann Intern Med. 2001;134:783–785. - PubMed
-
- Koller M, Heitmann K, Kussmann J, Lorenz W. Symptom reporting in cancer patients II: relations to social desirability, negative affect, and self-reported health behaviors. Cancer. 1999;86:1609–1620. - PubMed
-
- Given CW, Given B, Azzouz F, Kozachik S, Stommel M. Predictors of pain and fatigue in the year following diagnosis among elderly cancer patients. J Pain Symptom Manage. 2001;21:456–466. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

