Sialic acids as regulators of molecular and cellular interactions
- PMID: 19699080
- PMCID: PMC7127376
- DOI: 10.1016/j.sbi.2009.06.003
Sialic acids as regulators of molecular and cellular interactions
Abstract
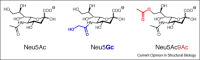
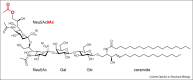
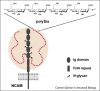
The wide occurrence of sialic acids (Sia) in various chemical forms linked as monomers or polymers in an outstanding position in a multitude of complex carbohydrates of animals and microorganisms renders them as most versatile function modulators in cell biology and pathology. A survey is presented of recent advances in the study of the influences that Sias have as bulky hydrophilic and electronegatively charged monosaccharides on animal cells and on their interaction with microorganisms. Some highlights are: sialylation leads to increased anti-inflammatory activity of IgG antibodies, facilitates the escape of microorganisms from the host's immune system, and in polymeric form is involved in the regulation of embryogenesis and neuronal growth and function. The role of siglecs in immunoregulation, the dynamics of lymphocyte binding to selectins and the interactions of toxins, viruses, and other microorganisms with the host's Sia are now better understood. N-Glycolylneuraminic acid from food is antigenic in man and seems to have pathogenic potential. Sia O-acetylation mediated by various eukaryotic and prokaryotic O-acetyltransferases modulates the affinity of these monosaccharides to mammalian and microbial receptors and hinders apoptosis. The functionally versatile O-acetylated ganglioside GD3 is an onco-fetal antigen.
Figures
References
-
- Kamerling J.P., Boons G.-J., Lee Y.C., Suzuki A., Taniguchi N., Voragen A.G.J., editors. vols. 1–4. Elsevier; Oxford: 2007. (Comprehensive Glycoscience—From Chemistry to Systems Biology).
-
This multiauthor, well-edited book series comprises all aspects of structure, analysis, chemical and biochemical synthesis, catabolism, molecular biology and cell biological, and pathophysiological aspects of complex carbohydrates. The reader's attention is drawn to the following chapters: Kobata A: Glycoprotein glycan structures, vol. 1, 39–72; Yu RK, Yanagisawa M, Ariga T: Glycosphingolipid structures, vol. 1, 73–122; Miyagi T, Yamaguchi K: Sialic acids, vol. 3, 297–323.
-
- Royle L., Matthews E., Corfield A., Berry M., Rudd P.M., Dwek R.A., Carrington S.D. Glycan structures of ocular surface mucins in man, rabbit and dog display species differences. Glycoconj J. 2008;25:763–773. - PubMed
-
- Janas T., Janas T. Polysialic acids: structure and properties. In: Dumitriu S., editor. Polysaccharides — Structural Diversity and Functional Versatility. edn 2. Marcel Dekker; New York: 2005. pp. 707–727.
-
- von Itzstein M., Thomson R. Anti-influenza drugs: the development of sialidase inhibitors. In: Kräusslich H.-G., Bartenschlager R., editors. vol. 189. Springer-Verlag; Berlin, Heidelberg: 2009. pp. 111–154. (Antiviral Strategies. Handbook Exp Pharmacol). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

