Deubiquitinase activities required for hepatocyte growth factor-induced scattering of epithelial cells
- PMID: 19699092
- PMCID: PMC2764384
- DOI: 10.1016/j.cub.2009.07.040
Deubiquitinase activities required for hepatocyte growth factor-induced scattering of epithelial cells
Abstract
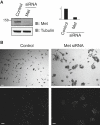
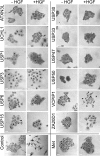
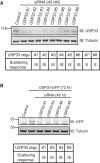
The scattering response of epithelial cells to activation of the Met receptor tyrosine kinase represents one facet of an "invasive growth" program. It is a complex event that incorporates loss of cell-cell adhesion, morphological changes, and cell motility. Ubiquitination is a reversible posttranslational modification that may target proteins for degradation or coordinate signal transduction pathways. There are approximately 79 active deubiquitinating enzymes (DUBs) predicted in the human genome. Here, via a small interfering RNA (siRNA) library approach, we have identified 12 DUBs that are necessary for aspects of the hepatocyte growth factor (HGF)-dependent scattering response of A549 cells. Different phenotypes are evident that range from full loss of scattering, similar to receptor knockdown (e.g., USP30, USP33, USP47), to loss of cell-cell contacts even in the absence of HGF but defective motility (e.g., USP3, ATXN3L). The knockdowns do not incur defective receptor, phosphatidylinositol 3-kinase, or MAP kinase activation. Our data suggest widespread involvement of the ubiquitin system at multiple stages of the Met activation response, implying significant crosstalk with phosphorylation-based transduction pathways. Development of small-molecule inhibitors of particular DUBs may offer a therapeutic approach to contain metastasis.
Figures

References
-
- Birchmeier C., Birchmeier W., Gherardi E., Vande Woude G.F. Met, metastasis, motility and more. Nat. Rev. Mol. Cell Biol. 2003;4:915–925. - PubMed
-
- Gentile A., Trusolino L., Comoglio P.M. The Met tyrosine kinase receptor in development and cancer. Cancer Metastasis Rev. 2008;27:85–94. - PubMed
-
- Hunter T. The age of crosstalk: Phosphorylation, ubiquitination, and beyond. Mol. Cell. 2007;28:730–738. - PubMed
-
- Welchman R.L., Gordon C., Mayer R.J. Ubiquitin and ubiquitin-like proteins as multifunctional signals. Nat. Rev. Mol. Cell Biol. 2005;6:599–609. - PubMed
-
- Scheel, H. (2005). Comparative analysis of the ubiquitin-proteasome system in Homo sapiens and Saccharomyces cerevisiae. PhD thesis, University of Cologne, Cologne, Germany.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

