Induction of autoimmunity by pristane and other naturally occurring hydrocarbons
- PMID: 19699150
- PMCID: PMC2746238
- DOI: 10.1016/j.it.2009.06.003
Induction of autoimmunity by pristane and other naturally occurring hydrocarbons
Abstract
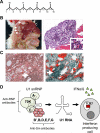
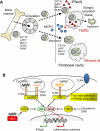
Tetramethylpentadecane (TMPD, or commonly known as pristane)-induced lupus is a murine model of systemic lupus erythematosus (SLE). Renal disease and autoantibody production strictly depend on signaling through the interferon (IFN)-I receptor. The major source of IFN-I is immature monocytes bearing high levels of the surface marker Ly6C. Interferon production is mediated exclusively by signaling through TLR7 and the adapter protein MyD88. It is likely that endogenous TLR7 ligands such as components of small nuclear ribonucleoprotein complexes are involved in triggering disease. Lupus autoantibodies are produced in ectopic lymphoid tissue developing in response to TMPD. This model is well suited for examining links between dysregulated IFN-I production and the pathogenesis of human SLE, which like TMPD-lupus, is associated with high levels of IFN-I.
Figures
Similar articles
-
TLR7-dependent and FcgammaR-independent production of type I interferon in experimental mouse lupus.J Exp Med. 2008 Dec 22;205(13):2995-3006. doi: 10.1084/jem.20080462. Epub 2008 Dec 1. J Exp Med. 2008. PMID: 19047436 Free PMC article.
-
Maintenance of autoantibody production in pristane-induced murine lupus.Arthritis Res Ther. 2015 Dec 30;17:384. doi: 10.1186/s13075-015-0886-9. Arthritis Res Ther. 2015. PMID: 26717913 Free PMC article.
-
Deficiency of the type I interferon receptor protects mice from experimental lupus.Arthritis Rheum. 2007 Nov;56(11):3770-83. doi: 10.1002/art.23023. Arthritis Rheum. 2007. PMID: 17968932 Free PMC article.
-
Pristane-induced lupus: considerations on this experimental model.Clin Rheumatol. 2017 Nov;36(11):2403-2414. doi: 10.1007/s10067-017-3811-6. Epub 2017 Sep 6. Clin Rheumatol. 2017. PMID: 28879482 Review.
-
Type I interferon in systemic lupus erythematosus.Curr Top Microbiol Immunol. 2007;316:359-86. doi: 10.1007/978-3-540-71329-6_17. Curr Top Microbiol Immunol. 2007. PMID: 17969456 Review.
Cited by
-
Requirements for innate immune pathways in environmentally induced autoimmunity.BMC Med. 2013 Apr 4;11:100. doi: 10.1186/1741-7015-11-100. BMC Med. 2013. PMID: 23557436 Free PMC article. Review.
-
Overexpression of membrane-bound fas ligand (CD95L) exacerbates autoimmune disease and renal pathology in pristane-induced lupus.J Immunol. 2013 Sep 1;191(5):2104-14. doi: 10.4049/jimmunol.1300341. Epub 2013 Aug 5. J Immunol. 2013. PMID: 23918976 Free PMC article.
-
Sphingolipids and Diagnosis, Prognosis, and Organ Damage in Systemic Lupus Erythematosus.Front Immunol. 2020 Sep 25;11:586737. doi: 10.3389/fimmu.2020.586737. eCollection 2020. Front Immunol. 2020. PMID: 33101319 Free PMC article. Review.
-
Fisetin inhibits pristine-induced systemic lupus erythematosus in a murine model through CXCLs regulation.Int J Mol Med. 2018 Dec;42(6):3220-3230. doi: 10.3892/ijmm.2018.3903. Epub 2018 Oct 1. Int J Mol Med. 2018. PMID: 30272314 Free PMC article.
-
Combinatorial therapeutic effect of resveratrol and piperine on murine model of systemic lupus erythematosus.Inflammopharmacology. 2020 Apr;28(2):401-424. doi: 10.1007/s10787-019-00662-w. Epub 2019 Nov 15. Inflammopharmacology. 2020. PMID: 31732838
References
-
- Avigan J, Blumer M. On the origin of pristane in marine organisms. J. Lipid Res. 1968;9:350–352. - PubMed
-
- Grob K, et al. Mineral oil material in canned foods. Food Additives & Contaminants. 1997;14:83–88. - PubMed
-
- Castle L, et al. Migration of mineral hydrocarbons into foods. 4. Waxed paper for packaging dry goods including bread, confectionary and for domestic use including microwave cooking. Food Additives and Contaminants. 1994;11:79–89. - PubMed
-
- Heimbach JT, et al. Dietary exposures to mineral hydrocarbons from food-use applications in the United States. Food Chem. Toxicol. 2002;40:555–571. - PubMed
-
- Cruickshank B. Follicular (mineral oil) lipidosis: I. Epidemiologic studies of involvement of the spleen. Hum. Pathol. 1984;15:724–730. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

