Loss of Kupffer cells in diet-induced obesity is associated with increased hepatic steatosis, STAT3 signaling, and further decreases in insulin signaling
- PMID: 19699298
- PMCID: PMC2763970
- DOI: 10.1016/j.bbadis.2009.08.007
Loss of Kupffer cells in diet-induced obesity is associated with increased hepatic steatosis, STAT3 signaling, and further decreases in insulin signaling
Abstract
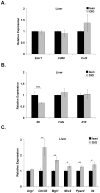
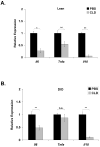
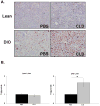
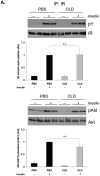
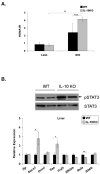
While adipose tissue-associated macrophages contribute to development of chronic inflammation and insulin resistance of obesity, little is known about the role of hepatic Kupffer cells in this environment. Here we address the impact of Kupffer cell ablation using clodronate-encapsulated liposome depletion in a diet-induced obese (DIO) and insulin resistant mouse model. Hepatic expression of macrophage markers measured by realtime RT-PCR remained unaltered in DIO mice despite characteristic expansion of adipose tissue-associated macrophages. DIO mouse livers displayed increased expression of alternative activation markers but unaltered proinflammatory cytokine expression when compared to lean mice. Kupffer cell ablation reduced hepatic anti-inflammatory cytokine IL-10 mRNA expression in lean and DIO mice by 95% and 84%, respectively. Despite decreased hepatic IL-6 gene expression after ablation in lean and DIO mice, hepatic STAT3 phosphorylation, Socs3 and acute phase protein mRNA expression increased. Kupffer cell ablation in DIO mice resulted in additional hepatic triglyceride accumulation and a 30-40% reduction in hepatic insulin receptor autophosphorylation and Akt activation. Implicating systemic loss of IL-10, high-fat-fed IL-10 knockout mice also displayed increased hepatic STAT3 signaling and hepatic triglyceride accumulation. Insulin signaling was not altered, however. In conclusion, Kupffer cells are a major source of hepatic IL-10 expression, the loss of which is associated with increased STAT3-dependent signaling and steatosis. One or more additional factors appear to be required, however, for the Kupffer cell-dependent protective effect on insulin receptor signaling in DIO mice.
Figures






Similar articles
-
Kupffer cell activation is a causal factor for hepatic insulin resistance.Am J Physiol Gastrointest Liver Physiol. 2010 Jan;298(1):G107-16. doi: 10.1152/ajpgi.00391.2009. Epub 2009 Oct 29. Am J Physiol Gastrointest Liver Physiol. 2010. PMID: 19875703
-
Clodronate liposomes improve metabolic profile and reduce visceral adipose macrophage content in diet-induced obese mice.PLoS One. 2011;6(9):e24358. doi: 10.1371/journal.pone.0024358. Epub 2011 Sep 12. PLoS One. 2011. PMID: 21931688 Free PMC article.
-
Inhibition of Gastric Inhibitory Polypeptide Receptor Signaling in Adipose Tissue Reduces Insulin Resistance and Hepatic Steatosis in High-Fat Diet-Fed Mice.Diabetes. 2017 Apr;66(4):868-879. doi: 10.2337/db16-0758. Epub 2017 Jan 17. Diabetes. 2017. PMID: 28096257
-
Fructose as a key player in the development of fatty liver disease.World J Gastroenterol. 2013 Feb 28;19(8):1166-72. doi: 10.3748/wjg.v19.i8.1166. World J Gastroenterol. 2013. PMID: 23482247 Free PMC article. Review.
-
Hepatoprotective and anti-inflammatory cytokines in alcoholic liver disease.J Gastroenterol Hepatol. 2012 Mar;27 Suppl 2(Suppl 2):89-93. doi: 10.1111/j.1440-1746.2011.07003.x. J Gastroenterol Hepatol. 2012. PMID: 22320924 Free PMC article. Review.
Cited by
-
Macrophage polarization and plasticity in health and disease.Immunol Res. 2012 Sep;53(1-3):11-24. doi: 10.1007/s12026-012-8291-9. Immunol Res. 2012. PMID: 22418728 Review.
-
Activated Kupffer cells inhibit insulin sensitivity in obese mice.FASEB J. 2015 Jul;29(7):2959-69. doi: 10.1096/fj.15-270496. Epub 2015 Mar 24. FASEB J. 2015. PMID: 25805830 Free PMC article.
-
Fructose and cardiometabolic disorders: the controversy will, and must, continue.Clinics (Sao Paulo). 2010 Jul;65(7):729-38. doi: 10.1590/S1807-59322010000700013. Clinics (Sao Paulo). 2010. PMID: 20668632 Free PMC article. Review.
-
Purple corn extract induces long-lasting reprogramming and M2 phenotypic switch of adipose tissue macrophages in obese mice.J Transl Med. 2019 Jul 23;17(1):237. doi: 10.1186/s12967-019-1972-6. J Transl Med. 2019. PMID: 31337415 Free PMC article.
-
Stress kinases in the development of liver steatosis and hepatocellular carcinoma.Mol Metab. 2021 Aug;50:101190. doi: 10.1016/j.molmet.2021.101190. Epub 2021 Feb 13. Mol Metab. 2021. PMID: 33588102 Free PMC article. Review.
References
-
- Trayhurn P, Wood IS. Adipokines: inflammation and the pleiotropic role of white adipose tissue. Br J Nutr. 2004;92:347–355. - PubMed
-
- Kern PA, Ranganathan S, Li C, Wood L, Ranganathan G. Adipose tissue tumor necrosis factor and interleukin-6 expression in human obesity and insulin resistance. Am J Physiol Endocrinol Metab. 2001;280:E745–751. - PubMed
-
- Bastard JP, Maachi M, Van Nhieu JT, Jardel C, Bruckert E, Grimaldi A, Robert JJ, Capeau J, Hainque B. Adipose tissue IL-6 content correlates with resistance to insulin activation of glucose uptake both in vivo and in vitro. J Clin Endocrinol Metab. 2002;87:2084–2089. - PubMed
-
- Senn JJ, Klover PJ, Nowak IA, Zimmers TA, Koniaris LG, Furlanetto RW, Mooney RA. Suppressor of cytokine signaling-3 (SOCS-3), a potential mediator of interleukin-6-dependent insulin resistance in hepatocytes. J Biol Chem. 2003;278:13740–13746. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

