Metal uptake by manganese superoxide dismutase
- PMID: 19699328
- PMCID: PMC2818121
- DOI: 10.1016/j.bbapap.2009.08.014
Metal uptake by manganese superoxide dismutase
Abstract
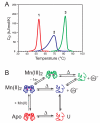
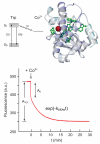
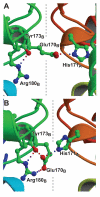
Manganese superoxide dismutase is an important antioxidant defense metalloenzyme that protects cells from damage by the toxic oxygen metabolite, superoxide free radical, formed as an unavoidable by-product of aerobic metabolism. Many years of research have gone into understanding how the metal cofactor interacts with small molecules in its catalytic role. In contrast, very little is presently known about how the protein acquires its metal cofactor, an important step in the maturation of the protein and one that is absolutely required for its biological function. Recent work is beginning to provide insight into the mechanisms of metal delivery to manganese superoxide dismutase in vivo and in vitro.
Copyright 2009 Elsevier B.V. All rights reserved.
Figures




Similar articles
-
In vitro metal uptake by recombinant human manganese superoxide dismutase.Arch Biochem Biophys. 2009 Nov;491(1-2):69-74. doi: 10.1016/j.abb.2009.09.003. Epub 2009 Sep 13. Arch Biochem Biophys. 2009. PMID: 19755112 Free PMC article.
-
Manganese superoxide dismutase in Saccharomyces cerevisiae acquires its metal co-factor through a pathway involving the Nramp metal transporter, Smf2p.J Biol Chem. 2001 Dec 14;276(50):47556-62. doi: 10.1074/jbc.M108923200. Epub 2001 Oct 15. J Biol Chem. 2001. PMID: 11602606
-
Regulation of an in vivo metal-exchangeable superoxide dismutase from Propionibacterium shermanii exhibiting activity with different metal cofactors.Biochem J. 1994 Dec 15;304 ( Pt 3)(Pt 3):803-8. doi: 10.1042/bj3040803. Biochem J. 1994. PMID: 7818484 Free PMC article.
-
The role of metals in production and scavenging of reactive oxygen species in photosystem II.Plant Cell Physiol. 2014 Jul;55(7):1224-32. doi: 10.1093/pcp/pcu053. Epub 2014 Apr 26. Plant Cell Physiol. 2014. PMID: 24771559 Review.
-
Superoxide dismutases-a review of the metal-associated mechanistic variations.Biochim Biophys Acta. 2010 Feb;1804(2):263-74. doi: 10.1016/j.bbapap.2009.11.005. Epub 2009 Nov 13. Biochim Biophys Acta. 2010. PMID: 19914406 Review.
Cited by
-
Superoxide dismutases: role in redox signaling, vascular function, and diseases.Antioxid Redox Signal. 2011 Sep 15;15(6):1583-606. doi: 10.1089/ars.2011.3999. Epub 2011 Jun 6. Antioxid Redox Signal. 2011. PMID: 21473702 Free PMC article. Review.
-
The SikCuZnSOD3 gene improves abiotic stress resistance in transgenic cotton.Mol Breed. 2021 Mar 10;41(3):26. doi: 10.1007/s11032-021-01217-0. eCollection 2021 Mar. Mol Breed. 2021. PMID: 37309423 Free PMC article.
-
Controlled hierarchical self-assembly of networked coordination nanocapsules via the use of molecular chaperones.Chem Sci. 2020 Oct 28;11(46):12547-12552. doi: 10.1039/d0sc05002d. Chem Sci. 2020. PMID: 34094454 Free PMC article.
-
Characterization of ancestral Fe/Mn superoxide dismutases indicates their cambialistic origin.Protein Sci. 2022 Oct;31(10):e4423. doi: 10.1002/pro.4423. Protein Sci. 2022. PMID: 36173172 Free PMC article.
-
The thermodynamics of protein interactions with essential first row transition metals.Biochim Biophys Acta. 2016 May;1860(5):879-891. doi: 10.1016/j.bbagen.2015.11.005. Epub 2015 Nov 10. Biochim Biophys Acta. 2016. PMID: 26569121 Free PMC article. Review.
References
-
- Bertini I, Gray HB, Stiefel EI, Valentine JS, editors. Biological Inorganic Chemistry: Structure and Reactivity. University Science Press; Sausalito: 2007.
-
- Bertini I, Rosato A. From genes to metalloproteins: A bioinformatic approach. Eur. J. Inorg. Chem. 2007;2007:2546–2555.
-
- Degtyarenko K. Metalloproteins. In: Dunn MJ, Jorde LB, Little PFR, Subramaniam S, editors. Encyclopedia of Genetics, Genomics, Proteomics and Bioinformatics, Part 3: Bioinformatics. John Wiley and Sons; New York: 2005.
-
- Eklund H, Uhlin U, Färnegårdh M, Logan DT, Nordlund P. Structure and function of the radical enzyme ribonucleotide reductase. Prog. Biophys. Mol. Biol. 2001;77:177–268. - PubMed
-
- Stubbe J, van der Donk W. Chem. Rev. 1998;98:2661–2662. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

