Quantification of extracellular UDP-galactose
- PMID: 19699703
- PMCID: PMC2790022
- DOI: 10.1016/j.ab.2009.08.022
Quantification of extracellular UDP-galactose
Abstract
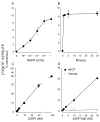
The human P2Y(14) receptor is potently activated by UDP-glucose (UDP-Glc), UDP-galactose (UDP-Gal), UDP-N-acetylglucosamine (UDP-GlcNAc), and UDP-glucuronic acid. Recently, cellular release of UDP-Glc and UDP-GlcNAc has been reported, but whether additional UDP-sugars are endogenous agonists for the P2Y(14) receptor remains poorly defined. In the present study, we describe an assay for the quantification of UDP-Gal with subnanomolar sensitivity. This assay is based on the enzymatic conversion of UDP-Gal to UDP, using 1-4-beta-galactosyltransferase. UDP is subsequently phosphorylated by nucleoside diphosphokinase in the presence of [gamma-(32)P]ATP and the formation of [gamma-(32)P]UTP is monitored by high-performance liquid chromatography. The overall conversion of UDP-Gal to [gamma-(32)P]UTP was linear between 0.5 and 30 nM UDP-Gal. Extracellular UDP-Gal was detected on resting cultures of various cell types, and increased release of UDP-Gal was observed in 1321N1 human astrocytoma cells stimulated with the protease-activated receptor agonist thrombin. The occurrence of regulated release of UDP-Gal suggests that, in addition to its role in glycosylation reactions, UDP-Gal is an important extracellular signaling molecule.
Figures



Similar articles
-
alpha-Lactalbumin (LA) stimulates milk beta-1,4-galactosyltransferase I (beta 4Gal-T1) to transfer glucose from UDP-glucose to N-acetylglucosamine. Crystal structure of beta 4Gal-T1 x LA complex with UDP-Glc.J Biol Chem. 2001 Oct 5;276(40):37665-71. doi: 10.1074/jbc.M102458200. Epub 2001 Aug 2. J Biol Chem. 2001. PMID: 11485999
-
Mechanism of UDP-sugar transport into intracellular vesicles. Occurrence of UDP-GlcNAc/UDP and UDP-Gal/UDP antiports.FEBS Lett. 1986 Nov 24;208(2):407-12. doi: 10.1016/0014-5793(86)81058-4. FEBS Lett. 1986. PMID: 3780976
-
Mutation of arginine 228 to lysine enhances the glucosyltransferase activity of bovine beta-1,4-galactosyltransferase I.Biochemistry. 2005 Mar 8;44(9):3202-10. doi: 10.1021/bi0479454. Biochemistry. 2005. PMID: 15736931
-
Fragment-based screening of the donor substrate specificity of human blood group B galactosyltransferase using saturation transfer difference NMR.J Biol Chem. 2006 Oct 27;281(43):32728-40. doi: 10.1074/jbc.M600424200. Epub 2006 Aug 21. J Biol Chem. 2006. PMID: 16923820
-
Regulated release of nucleotides and UDP sugars from astrocytoma cells.Novartis Found Symp. 2006;276:73-84; discussion 84-90, 107-12, 275-81. Novartis Found Symp. 2006. PMID: 16805424 Review.
Cited by
-
Signalling and pharmacological properties of the P2Y receptor.Acta Physiol (Oxf). 2010 Jun;199(2):149-60. doi: 10.1111/j.1748-1716.2010.02116.x. Epub 2010 Mar 24. Acta Physiol (Oxf). 2010. PMID: 20345417 Free PMC article. Review.
-
UDP-glucose promotes neutrophil recruitment in the lung.Purinergic Signal. 2016 Dec;12(4):627-635. doi: 10.1007/s11302-016-9524-5. Epub 2016 Jul 15. Purinergic Signal. 2016. PMID: 27421735 Free PMC article.
-
Role of UDP-Sugar Receptor P2Y14 in Murine Osteoblasts.Int J Mol Sci. 2020 Apr 15;21(8):2747. doi: 10.3390/ijms21082747. Int J Mol Sci. 2020. PMID: 32326617 Free PMC article.
-
UDP-Sugars as Extracellular Signaling Molecules: Cellular and Physiologic Consequences of P2Y14 Receptor Activation.Mol Pharmacol. 2015 Jul;88(1):151-60. doi: 10.1124/mol.115.098756. Epub 2015 Mar 31. Mol Pharmacol. 2015. PMID: 25829059 Free PMC article. Review.
-
Identification of a carbohydrate recognition motif of purinergic receptors.Elife. 2023 Nov 13;12:e85449. doi: 10.7554/eLife.85449. Elife. 2023. PMID: 37955640 Free PMC article.
References
-
- Burnstock G, Williams M. P2 purinergic receptors: modulation of cell function and therapeutic potential. J Pharmacol Exp Ther. 2000;295:862–869. - PubMed
-
- Chambers JK, Macdonald LE, Sarau HM, Ames RS, Freeman K, Foley JJ, Zhu Y, McLaughlin MM, Murdock P, McMillan L, Trill J, Swift A, Aiyar N, Taylor P, Vawter L, Naheed S, Szekeres P, Hervieu G, Scott C, Watson JM, Murphy AJ, Duzic E, Klein C, Bergsma DJ, Wilson S, Livi GP. A G protein-coupled receptor for UDP-glucose. J Biol Chem. 2000;275:10767–10771. - PubMed
-
- Abbracchio MP, Boeynaems JM, Barnard EA, Boyer JL, Kennedy C, Miras-Portugal MT, King BF, Gachet C, Jacobson KA, Weisman GA, Burnstock G. Characterization of the UDP-glucose receptor (re-named here the P2Y(14) receptor) adds diversity to the P2Y receptor family. Trends Pharmacol Sci. 2003;24:52–55. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

