Tgfbr2 is required for development of the skull vault
- PMID: 19699732
- PMCID: PMC2753698
- DOI: 10.1016/j.ydbio.2009.08.015
Tgfbr2 is required for development of the skull vault
Abstract
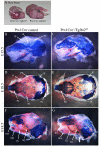
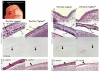
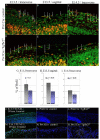
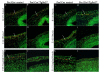
Transforming growth factor beta (TGFbeta) is known to play important roles in multiple developmental processes. One of the main functions is in skeletal development. Our previous studies demonstrated that loss of Tgfbr2 in Prx1Cre-expressing limb mesenchyme results in defects in the long bones and joints of mice. Here we show that loss of Tgfbr2 also results in defects in the development of the skull vault indicating Tgfbr2 has a critical role in intramembranous bone formation as well as endochondral bone formation. Mutant mice did not survive after birth and demonstrated an open skull. The first signs of skull defects were observed at E14.5 day. Prx1Cre(+)/Tgfbr2(f/f) embryos showed significantly reduced cell proliferation in the developing mesenchyme of the skull by E14.5 day without any detectable alteration in apoptosis suggesting that reduced cell proliferation in Prx1Cre(+)/Tgfbr2(f/f) embryos was at least partially responsible for the defects observed. Immunofluorescent staining showed a significant reduction in the expression of Runx2/Cbfa1 and Osterix/Sp7 in Prx1Cre(+)/Tgfbr2(f/f) embryos suggesting that osteoblast differentiation was also altered in Prx1Cre(+)/Tgfbr2(f/f) embryos. To distinguish between the effects of losing Tgfbr2 on mesenchymal proliferation versus osteoblast differentiation, osteoprogenitor cells from the skulls of Tgfbr2(f/f) embryos were cultured under conditions of high cell density and Tgfbr2 was deleted from the cells using Adeno-Cre virus. RT-PCR analysis showed that the mRNA level of Runx2 and Osterix as well as Dlx5 and Msx2 were down-regulated in Tgfbr2-deleted cultures compared to control cultures indicating that Tgfbr2 regulates osteoblast differentiation independent of regulating proliferation. Together, these results suggest that Tgfbr2 is required for normal development of the skull.
Figures

References
-
- Acampora D, Merlo GR, Paleari L, Zerega B, Postiglione MP, Mantero S, Bober E, Barbieri O, Simeone A, Levi G. Craniofacial, vestibular and bone defects in mice lacking the Distal-less-related gene Dlx5. Development. 1999;126:3795–809. - PubMed
-
- Baffi MO, Slattery E, Sohn P, Moses HL, Chytil A, Serra R. Conditional deletion of the TGF-beta type II receptor in Col2a expressing cells results in defects in the axial skeleton without alterations in chondrocyte differentiation or embryonic development of long bones. Dev Biol. 2004;276:124–42. - PubMed
-
- Celil AB, Hollinger JO, Campbell PG. Osx transcriptional regulation is mediated by additional pathways to BMP2/Smad signaling. J Cell Biochem. 2005;95:518–28. - PubMed
-
- Chai Y, Jiang X, Ito Y, Bringas P, Jr., Han J, Rowitch DH, Soriano P, McMahon AP, Sucov HM. Fate of the mammalian cranial neural crest during tooth and mandibular morphogenesis. Development. 2000;127:1671–9. - PubMed
-
- Chytil A, Magnuson MA, Wright CV, Moses HL. Conditional inactivation of the TGF-beta type II receptor using Cre:Lox. Genesis. 2002;32:73–5. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

