BCL9 is an essential component of canonical Wnt signaling that mediates the differentiation of myogenic progenitors during muscle regeneration
- PMID: 19699733
- PMCID: PMC3259687
- DOI: 10.1016/j.ydbio.2009.08.014
BCL9 is an essential component of canonical Wnt signaling that mediates the differentiation of myogenic progenitors during muscle regeneration
Abstract
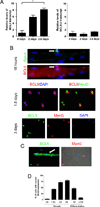
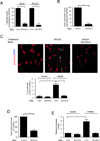
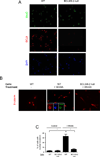
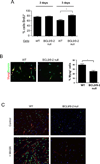
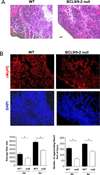
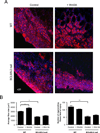
Muscle stem cells and their progeny play a fundamental role in the regeneration of adult skeletal muscle. We have previously shown that activation of the canonical Wnt/beta-catenin signaling pathway in adult myogenic progenitors is required for their transition from rapidly dividing transient amplifying cells to more differentiated progenitors. Whereas Wnt signaling in Drosophila is dependent on the presence of the co-regulator Legless, previous studies of the mammalian ortholog of Legless, BCL9 (and its homolog, BCL9-2), have not revealed an essential role of these proteins in Wnt signaling in specific tissues during development. Using Cre-lox technology to delete BCL9 and BCL9-2 in the myogenic lineage in vivo and RNAi technology to knockdown the protein levels in vitro, we show that BCL9 is required for activation of the Wnt/beta-catenin cascade in adult mammalian myogenic progenitors. We observed that the nuclear localization of beta-catenin and downstream TCF/LEF-mediated transcription, which are normally observed in myogenic progenitors upon addition of exogenous Wnt and during muscle regeneration, were abrogated when BCL9/9-2 levels were reduced. Furthermore, reductions of BCL9/9-2 inhibited the promotion of myogenic differentiation by Wnt and the normal regenerative response of skeletal muscle. These results suggest a critical role of BCL9/9-2 in the Wnt-mediated regulation of adult, as opposed to embryonic, myogenic progenitors.
Figures


References
-
- Anakwe K, Robson L, Hadley J, Buxton P, Church V, Allen S, et al. Wnt signalling regulates myogenic differentiation in the developing avian wing. Development. 2003;130:3503–3514. - PubMed
-
- Belenkaya TY, Han C, Standley HJ, Lin X, Houston DW, Heasman J, Lin X. pygopus Encodes a nuclear protein essential for wingless/Wnt signaling. Development. 2002;129:4089–4101. - PubMed
-
- Borello U, Berarducci B, Murphy P, Bajard L, Buffa V, Piccolo S, et al. The Wnt/beta-catenin pathway regulates Gli-mediated Myf5 expression during somitogenesis. Development. 2006;133:3723–3732. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

