Different actions of ecdysis-triggering hormone on the brain and ventral nerve cord of the hornworm, Manduca sexta
- PMID: 19699740
- PMCID: PMC2823964
- DOI: 10.1016/j.ygcen.2009.08.008
Different actions of ecdysis-triggering hormone on the brain and ventral nerve cord of the hornworm, Manduca sexta
Abstract
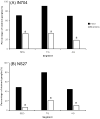
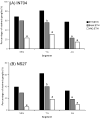
Ecdysis, or the shedding of the old cuticle, depends on coordinated stereotyped behaviors, regulated by a number of neuropeptides. In the hornworm, Manduca sexta, two neuropeptides interact, namely ecdysis-triggering hormone (ETH) and eclosion hormone. We looked at the effects of ETH in vivo and in vitro, on the brain and the ventral nerve cord to determine the roles played by these hormones. We monitored ecdysis onset and the presence of cGMP and eclosion hormone immunoreactivity. In vivo, only a fraction of larvae lacking the cell bodies containing eclosion hormone, and injected with ETH, were able to undergo ecdysis, with a delayed response. These animals showed strongest cGMP immunoreactivity in the subesophageal and thoracic ganglia, with concomitant reductions in eclosion hormone immunoreactivity in descending axons in comparison with animals not undergoing ecdysis. Animals lacking the brain showed reduced to no cGMP levels in all ganglia. In vitro, isolated CNS preparations lacking the brain initiated ecdysis motor programs after incubation in ETH, with faster onset times than controls, and with reduced cGMP immunoreactivity. If ETH was applied only to the brain of the isolated CNS, cGMP immunoreactivity was noted primarily in the subesophageal and thoracic ganglia, with a decrease in eclosion hormone immunoreactivity in descending axons. ETH addition to the rest of the nerve cord showed reduced eclosion hormone immunoreactivity but little to no cGMP immunoreactivity in any ganglion. Controls showed strong cGMP immunoreactivity in all ganglia, and even greater reductions in eclosion hormone staining after ETH application. These results support previous suggestions that eclosion hormone is required for a positive feedback loop with ETH as well as onset of an inhibitory component, but also suggest that ETH stimulates eclosion hormone release at multiple spike initiation zones. The resultant up regulation of cGMP does not appear to be required for onset of ecdysis. A new model for ecdysis regulation is considered.
Copyright 2009 Elsevier Inc. All rights reserved.
Figures

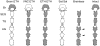
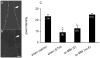


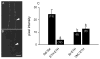

References
-
- Adams ME, Zitnan D. Identification of ecdysis-triggering hormone in the silkworm Bombyx mori. Biochem Biophys Res Commun. 1997;230:188–191. - PubMed
-
- Calabrese RL. Control of multiple impulse-initiation sites in a leech intemeuron. J Neurophysiol. 1980;44:878–896. - PubMed
-
- Copenhaver PF, Truman JW. The role of eclosion hormone in the larval ecdyses of Manduca Sexta. J Insect Physiol. 1982;28:695–701.
-
- Davis NT, Homberg U, Dircksen H, Levine RB, Hildebrand JG. Crustacean cardioactive peptide-immunoreactive neurons in the hawkmoth Manduca sexta and changes in their immunoreactivity during postembryonic development. J Comp Neurol. 1993;338:612–627. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

