Receptor-selective agonists induce emesis and Fos expression in the brain and enteric nervous system of the least shrew (Cryptotis parva)
- PMID: 19699757
- PMCID: PMC2771428
- DOI: 10.1016/j.pbb.2009.08.010
Receptor-selective agonists induce emesis and Fos expression in the brain and enteric nervous system of the least shrew (Cryptotis parva)
Abstract
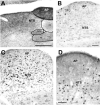
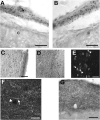
Research on the mechanisms of emesis has implicated multiple neurotransmitters via both central (dorsal vagal complex) and peripheral (enteric neurons and enterochromaffin cells) anatomical substrates. Taking advantage of advances in receptor-specific agonists, and utilizing Fos expression as a functional activity marker, this study demonstrates a strong, but incomplete, overlap in anatomical substrates for a variety of emetogens. We used cisplatin and specific agonists to 5-HT(3) serotonergic, D(2)/D(3) dopaminergic, and NK(1) tachykininergic receptors to induce vomiting in the least shrew (Cryptotis parva), and quantified the resulting Fos expression. The least shrew is a small mammal whose responses to emetic challenges are very similar to its human counterparts. In all cases, the enteric nervous system, nucleus of the solitary tract, and dorsal motor nucleus of the vagus demonstrated significantly increased Fos immunoreactivity (Fos-IR). However, Fos-IR induction was notably absent from the area postrema following the dopaminergic and NK(1) receptor-specific agents. Two brain nuclei not usually discussed regarding emesis, the dorsal raphe nucleus and paraventricular thalamic nucleus, also demonstrated increased emesis-related Fos-IR. Taken together, these data suggest the dorsal vagal complex is part of a common pathway for a variety of distinct emetogens, but there are central emetic substrates, both medullary and diencephalic, that can be accessed without directly stimulating the area postrema.
Figures
Similar articles
-
Delta 9-tetrahydrocannabinol suppresses vomiting behavior and Fos expression in both acute and delayed phases of cisplatin-induced emesis in the least shrew.Behav Brain Res. 2009 Jan 3;196(1):30-6. doi: 10.1016/j.bbr.2008.07.028. Epub 2008 Jul 31. Behav Brain Res. 2009. PMID: 18721829 Free PMC article.
-
Utilization of the least shrew as a rapid and selective screening model for the antiemetic potential and brain penetration of substance P and NK1 receptor antagonists.Brain Res. 2008 Jun 12;1214:58-72. doi: 10.1016/j.brainres.2008.03.077. Epub 2008 Apr 9. Brain Res. 2008. PMID: 18471804 Free PMC article.
-
Chemotherapy agent cisplatin induces 48-h Fos expression in the brain of a vomiting species, the house musk shrew (Suncus murinus).Am J Physiol Regul Integr Comp Physiol. 2009 Apr;296(4):R902-11. doi: 10.1152/ajpregu.90952.2008. Epub 2009 Feb 18. Am J Physiol Regul Integr Comp Physiol. 2009. PMID: 19225146 Free PMC article.
-
Roles of substance P and NK(1) receptor in the brainstem in the development of emesis.J Pharmacol Sci. 2003 Feb;91(2):87-94. doi: 10.1254/jphs.91.87. J Pharmacol Sci. 2003. PMID: 12686752 Review.
-
[Role of serotonin in emesis].Nihon Yakurigaku Zasshi. 1996 Nov;108(5):233-42. doi: 10.1254/fpj.108.233. Nihon Yakurigaku Zasshi. 1996. PMID: 8974084 Review. Japanese.
Cited by
-
A Comparative Study of the Antiemetic Effects of α2-Adrenergic Receptor Agonists Clonidine and Dexmedetomidine against Diverse Emetogens in the Least Shrew (Cryptotis parva) Model of Emesis.Int J Mol Sci. 2024 Apr 23;25(9):4603. doi: 10.3390/ijms25094603. Int J Mol Sci. 2024. PMID: 38731821 Free PMC article.
-
Differential activation of pontomedullary nuclei by acid perfusion of different regions of the esophagus.Brain Res. 2010 Sep 17;1352:94-107. doi: 10.1016/j.brainres.2010.07.048. Epub 2010 Jul 22. Brain Res. 2010. PMID: 20655885 Free PMC article.
-
A re-consideration of neural/receptor mechanisms in chemotherapy-induced nausea and vomiting: current scenario and future perspective.Pharmacol Rep. 2023 Oct;75(5):1126-1137. doi: 10.1007/s43440-023-00514-z. Epub 2023 Aug 16. Pharmacol Rep. 2023. PMID: 37584820 Review.
-
Neurokinin-1 Antagonists for Postoperative Nausea and Vomiting.Drugs. 2021 Jul;81(10):1171-1179. doi: 10.1007/s40265-021-01532-y. Epub 2021 Jun 9. Drugs. 2021. PMID: 34106456 Review.
-
The Contribution of Phospholipase C in Vomiting in the Least Shrew (Cryptotis Parva) Model of Emesis.Front Pharmacol. 2021 Sep 10;12:736842. doi: 10.3389/fphar.2021.736842. eCollection 2021. Front Pharmacol. 2021. PMID: 34566660 Free PMC article.
References
-
- Andrews PL. Physiology of nausea and vomiting. Br J Anaesth. 1992;69:2S–19S. - PubMed
-
- Arluison M, Derer P. Forebrain connections of the rat paraventricular thalamic nucleus as demonstrated using the carbocyanide dye DiI. Neurobiology (Bp) 1993;1:337–50. - PubMed
-
- Berthoud HR, Patterson LM, Zheng H. Vagal-enteric interface: vagal activation-induced expression of c-Fos and p-CREB in neurons of the upper gastrointestinal tract and pancreas. Anat Rec. 2001;262:29–40. - PubMed
-
- Bhandari P, Bingham S, Andrews PL. The neuropharmacology of loperamide-induced emesis in the ferret: the role of the area postrema, vagus, opiate and 5-HT3 receptors. Neuropharmacology. 1992;31:735–42. - PubMed
-
- Boissonade FM, Davison JS, Egizii R, Lucier GE, Sharkey KA. The dorsal vagal complex of the ferret: anatomical and immunohistochemical studies. Neurogastroenterol Motil. 1996;8:255–72. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

