Structural model for phenylalkylamine binding to L-type calcium channels
- PMID: 19700404
- PMCID: PMC2788883
- DOI: 10.1074/jbc.M109.027326
Structural model for phenylalkylamine binding to L-type calcium channels
Abstract
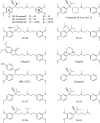
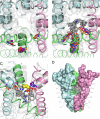
Phenylalkylamines (PAAs), a major class of L-type calcium channel (LTCC) blockers, have two aromatic rings connected by a flexible chain with a nitrile substituent. Structural aspects of ligand-channel interactions remain unclear. We have built a KvAP-based model of LTCC and used Monte Carlo energy minimizations to dock devapamil, verapamil, gallopamil, and other PAAs. The PAA-LTCC models have the following common features: (i) the meta-methoxy group in ring A, which is proximal to the nitrile group, accepts an H-bond from a PAA-sensing Tyr_IIIS6; (ii) the meta-methoxy group in ring B accepts an H-bond from a PAA-sensing Tyr_IVS6; (iii) the ammonium group is stabilized at the focus of P-helices; and (iv) the nitrile group binds to a Ca(2+) ion coordinated by the selectivity filter glutamates in repeats III and IV. The latter feature can explain Ca(2+) potentiation of PAA action and the presence of an electronegative atom at a similar position of potent PAA analogs. Tyr substitution of a Thr in IIIS5 is known to enhance action of devapamil and verapamil. Our models predict that the para-methoxy group in ring A of devapamil and verapamil accepts an H-bond from this engineered Tyr. The model explains structure-activity relationships of PAAs, effects of LTCC mutations on PAA potency, data on PAA access to LTCC, and Ca(2+) potentiation of PAA action. Common and class-specific aspects of action of PAAs, dihydropyridines, and benzothiazepines are discussed in view of the repeat interface concept.
Figures


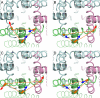

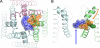
Similar articles
-
Opposite effects of a single IIIS5 mutation on phenylalkylamine and dihydropyridine interaction with L-type Ca2+ channels.J Biol Chem. 2004 Dec 31;279(53):55211-7. doi: 10.1074/jbc.M409008200. Epub 2004 Oct 25. J Biol Chem. 2004. PMID: 15504730
-
Structural model for dihydropyridine binding to L-type calcium channels.J Biol Chem. 2009 Jul 10;284(28):19006-17. doi: 10.1074/jbc.M109.011296. Epub 2009 May 5. J Biol Chem. 2009. PMID: 19416978 Free PMC article.
-
Phenylalkylamines in calcium channels: computational analysis of experimental structures.J Comput Aided Mol Des. 2020 Nov;34(11):1157-1169. doi: 10.1007/s10822-020-00330-0. Epub 2020 Jul 10. J Comput Aided Mol Des. 2020. PMID: 32648151
-
1,4-Dihydropyridine scaffold in medicinal chemistry, the story so far and perspectives (part 1): action in ion channels and GPCRs.Curr Med Chem. 2011;18(32):4901-22. doi: 10.2174/092986711797535173. Curr Med Chem. 2011. PMID: 22050742 Review.
-
Molecular determinants of drug binding and action on L-type calcium channels.Annu Rev Pharmacol Toxicol. 1997;37:361-96. doi: 10.1146/annurev.pharmtox.37.1.361. Annu Rev Pharmacol Toxicol. 1997. PMID: 9131258 Review.
Cited by
-
P-Loop Channels: Experimental Structures, and Physics-Based and Neural Networks-Based Models.Membranes (Basel). 2022 Feb 16;12(2):229. doi: 10.3390/membranes12020229. Membranes (Basel). 2022. PMID: 35207150 Free PMC article. Review.
-
Functional, electrophysiological and molecular docking analysis of the modulation of Cav 1.2 channels in rat vascular myocytes by murrayafoline A.Br J Pharmacol. 2016 Jan;173(2):292-304. doi: 10.1111/bph.13369. Epub 2015 Dec 23. Br J Pharmacol. 2016. PMID: 26493241 Free PMC article.
-
Seeking the exclusive binding region of phenylalkylamine derivatives on human T-type calcium channels via homology modeling and molecular dynamics simulation approach.Pharmacol Res Perspect. 2021 May;9(3):e00783. doi: 10.1002/prp2.783. Pharmacol Res Perspect. 2021. PMID: 33984189 Free PMC article.
-
Ca²⁺ channels and praziquantel: a view from the free world.Parasitol Int. 2013 Dec;62(6):619-28. doi: 10.1016/j.parint.2012.12.001. Epub 2012 Dec 16. Parasitol Int. 2013. PMID: 23246536 Free PMC article. Review.
-
Identification of new batrachotoxin-sensing residues in segment IIIS6 of the sodium channel.J Biol Chem. 2011 Apr 15;286(15):13151-60. doi: 10.1074/jbc.M110.208496. Epub 2011 Feb 8. J Biol Chem. 2011. PMID: 21303907 Free PMC article.
References
-
- Hockerman G. H., Peterson B. Z., Johnson B. D., Catterall W. A. (1997) Annu. Rev. Pharmacol. Toxicol. 37, 361–396 - PubMed
-
- Lacinova L. (2005) Gen. Physiol. Biophys. 24, Suppl. 1, 1–78 - PubMed
-
- Hering S., Savchenko A., Strübing C., Lakitsch M., Striessnig J. (1993) Mol. Pharmacol. 43, 820–826 - PubMed
-
- Seydl K., Kimball D., Schindler H., Romanin C. (1993) Pflugers Arch. 424, 552–554 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

