CYP704B1 is a long-chain fatty acid omega-hydroxylase essential for sporopollenin synthesis in pollen of Arabidopsis
- PMID: 19700560
- PMCID: PMC2754625
- DOI: 10.1104/pp.109.144469
CYP704B1 is a long-chain fatty acid omega-hydroxylase essential for sporopollenin synthesis in pollen of Arabidopsis
Abstract
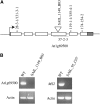
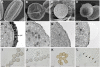
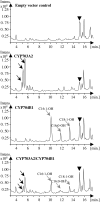
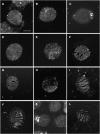
Sporopollenin is the major component of the outer pollen wall (exine). Fatty acid derivatives and phenolics are thought to be its monomeric building blocks, but the precise structure, biosynthetic route, and genetics of sporopollenin are poorly understood. Based on a phenotypic mutant screen in Arabidopsis (Arabidopsis thaliana), we identified a cytochrome P450, designated CYP704B1, as being essential for exine development. CYP704B1 is expressed in the developing anthers. Mutations in CYP704B1 result in impaired pollen walls that lack a normal exine layer and exhibit a characteristic striped surface, termed zebra phenotype. Heterologous expression of CYP704B1 in yeast cells demonstrated that it catalyzes omega-hydroxylation of long-chain fatty acids, implicating these molecules in sporopollenin synthesis. Recently, an anther-specific cytochrome P450, denoted CYP703A2, that catalyzes in-chain hydroxylation of lauric acid was also shown to be involved in sporopollenin synthesis. This shows that different classes of hydroxylated fatty acids serve as essential compounds for sporopollenin formation. The genetic relationships between CYP704B1, CYP703A2, and another exine gene, MALE STERILITY2, which encodes a fatty acyl reductase, were explored. Mutations in all three genes resulted in pollen with remarkably similar zebra phenotypes, distinct from those of other known exine mutants. The double and triple mutant combinations did not result in the appearance of novel phenotypes or enhancement of single mutant phenotypes. This implies that each of the three genes is required to provide an indispensable subset of fatty acid-derived components within the sporopollenin biosynthesis framework.
Figures






Similar articles
-
CYP703 is an ancient cytochrome P450 in land plants catalyzing in-chain hydroxylation of lauric acid to provide building blocks for sporopollenin synthesis in pollen.Plant Cell. 2007 May;19(5):1473-87. doi: 10.1105/tpc.106.045948. Epub 2007 May 11. Plant Cell. 2007. PMID: 17496121 Free PMC article.
-
LAP5 and LAP6 encode anther-specific proteins with similarity to chalcone synthase essential for pollen exine development in Arabidopsis.Plant Physiol. 2010 Jul;153(3):937-55. doi: 10.1104/pp.110.157446. Epub 2010 May 4. Plant Physiol. 2010. PMID: 20442277 Free PMC article.
-
A large-scale genetic screen in Arabidopsis to identify genes involved in pollen exine production.Plant Physiol. 2011 Oct;157(2):947-70. doi: 10.1104/pp.111.179523. Epub 2011 Aug 17. Plant Physiol. 2011. PMID: 21849515 Free PMC article.
-
Genetic regulation of sporopollenin synthesis and pollen exine development.Annu Rev Plant Biol. 2011;62:437-60. doi: 10.1146/annurev-arplant-042809-112312. Annu Rev Plant Biol. 2011. PMID: 21275644 Review.
-
The biosynthesis, composition and assembly of the outer pollen wall: A tough case to crack.Phytochemistry. 2015 May;113:170-82. doi: 10.1016/j.phytochem.2014.05.002. Epub 2014 Jun 3. Phytochemistry. 2015. PMID: 24906292 Review.
Cited by
-
Sporopollenin biosynthetic enzymes interact and constitute a metabolon localized to the endoplasmic reticulum of tapetum cells.Plant Physiol. 2013 Jun;162(2):616-25. doi: 10.1104/pp.112.213124. Epub 2013 Apr 30. Plant Physiol. 2013. PMID: 23632852 Free PMC article.
-
FlowerNet: a gene expression correlation network for anther and pollen development.Plant Physiol. 2015 Apr;167(4):1717-30. doi: 10.1104/pp.114.253807. Epub 2015 Feb 9. Plant Physiol. 2015. PMID: 25667314 Free PMC article.
-
Comparison of the Micromorphology and Ultrastructure of Pollen Grains of Selected Rubus idaeus L. Cultivars Grown in Commercial Plantation.Plants (Basel). 2020 Sep 12;9(9):1194. doi: 10.3390/plants9091194. Plants (Basel). 2020. PMID: 32932712 Free PMC article.
-
ATP-binding cassette transporter G26 is required for male fertility and pollen exine formation in Arabidopsis.Plant Physiol. 2010 Oct;154(2):678-90. doi: 10.1104/pp.110.161968. Epub 2010 Aug 23. Plant Physiol. 2010. PMID: 20732973 Free PMC article.
-
Analysis of Pollen Allergens in Lily by Transcriptome and Proteome Data.Int J Mol Sci. 2019 Nov 24;20(23):5892. doi: 10.3390/ijms20235892. Int J Mol Sci. 2019. PMID: 31771269 Free PMC article.
References
-
- Aarts MG, Dirkse WG, Stiekema WJ, Pereira A (1993) Transposon tagging of a male sterility gene in Arabidopsis. Nature 363: 715–717 - PubMed
-
- Aarts MG, Hodge R, Kalantidis K, Florack D, Wilson ZA, Mulligan BJ, Stiekema WJ, Scott R, Pereira A (1997) The Arabidopsis MALE STERILITY 2 protein shares similarity with reductases in elongation/condensation complexes. Plant J 12: 615–623 - PubMed
-
- Ahlers F, Thom I, Lambert J, Kuckuk R, Wiermann R (1999) 1H NMR analysis of sporopollenin from Typha angustifolia. Phytochemistry 5: 1095–1098
-
- Albertsen MC, Fox T, Huffmann G, Trimnell M (2006) Nucleotide sequences affecting plant male fertility and methods of using same. U.S. Patent 7,098,388 B2, Pioneer Hi-Bred International
-
- Alonso JM, Stepanova AN, Leisse TJ, Kim CJ, Chen H, Shinn P, Stevenson DK, Zimmerman J, Barajas P, Cheuk R, et al (2003) Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301: 653–657 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

