Mechanism-based inactivation of CYP2B1 and its F-helix mutant by two tert-butyl acetylenic compounds: covalent modification of prosthetic heme versus apoprotein
- PMID: 19700628
- PMCID: PMC2775271
- DOI: 10.1124/jpet.109.158782
Mechanism-based inactivation of CYP2B1 and its F-helix mutant by two tert-butyl acetylenic compounds: covalent modification of prosthetic heme versus apoprotein
Abstract
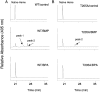
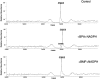
The mechanism-based inactivation of cytochrome CYP2B1 [wild type (WT)] and its Thr205 to Ala mutant (T205A) by tert-butylphenylacetylene (BPA) and tert-butyl 1-methyl-2-propynyl ether (BMP) in the reconstituted system was investigated. The inactivation of WT by BPA exhibited a k(inact)/K(I) value of 1343 min(-1)mM(-1) and a partition ratio of 1. The inactivation of WT by BMP exhibited a k(inact)/K(I) value of 33 min(-1)mM(-1) and a partition ratio of 10. Liquid chromatography/tandem mass spectrometry analysis (LC/MS/MS) of the WT revealed 1) inactivation by BPA resulted in the formation of a protein adduct with a mass increase equivalent to the mass of BPA plus one oxygen atom, and 2) inactivation by BMP resulted in the formation of multiple heme adducts that all exhibited a mass increase equivalent to BMP plus one oxygen atom. LC/MS/MS analysis indicated the formation of glutathione (GSH) conjugates by the reaction of GSH with the ethynyl moiety of BMP or BPA with the oxygen being added to the internal or terminal carbon. For the inactivation of T205A by BPA and BMP, the k(inact)/K(I) values were suppressed by 100- and 4-fold, respectively, and the partition ratios were increased 9- and 3.5-fold, respectively. Only one major heme adduct was detected following the inactivation of the T205A by BMP. These results show that the Thr205 in the F-helix plays an important role in the efficiency of the mechanism-based inactivation of CYP2B1 by BPA and BMP. Homology modeling and substrate docking studies were presented to facilitate the interpretation of the experimental results.
Figures
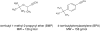
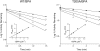



Similar articles
-
Targeting of the highly conserved threonine 302 residue of cytochromes P450 2B family during mechanism-based inactivation by aryl acetylenes.Arch Biochem Biophys. 2011 Mar 1;507(1):135-43. doi: 10.1016/j.abb.2010.09.006. Epub 2010 Sep 15. Arch Biochem Biophys. 2011. PMID: 20836985 Free PMC article. Review.
-
Mechanism-based inactivation of cytochromes P450 2E1 and 2E1 T303A by tert-butyl acetylenes: characterization of reactive intermediate adducts to the heme and apoprotein.Chem Res Toxicol. 2002 Dec;15(12):1561-71. doi: 10.1021/tx020052x. Chem Res Toxicol. 2002. PMID: 12482238
-
Covalent modification of Thr302 in cytochrome P450 2B1 by the mechanism-based inactivator 4-tert-butylphenylacetylene.J Pharmacol Exp Ther. 2010 Jun;333(3):663-9. doi: 10.1124/jpet.109.164350. Epub 2010 Mar 3. J Pharmacol Exp Ther. 2010. PMID: 20200115 Free PMC article.
-
The inactivation of cytochrome P450 3A5 by 17alpha-ethynylestradiol is cytochrome b5-dependent: metabolic activation of the ethynyl moiety leads to the formation of glutathione conjugates, a heme adduct, and covalent binding to the apoprotein.J Pharmacol Exp Ther. 2007 Apr;321(1):276-87. doi: 10.1124/jpet.106.117861. Epub 2007 Jan 24. J Pharmacol Exp Ther. 2007. PMID: 17251390
-
Acetylenes: cytochrome P450 oxidation and mechanism-based enzyme inactivation.Drug Metab Rev. 2019 May;51(2):162-177. doi: 10.1080/03602532.2019.1632891. Epub 2019 Jul 7. Drug Metab Rev. 2019. PMID: 31203694 Free PMC article. Review.
Cited by
-
Mechanism-based inactivation of human cytochrome P450 2B6 by clopidogrel: involvement of both covalent modification of cysteinyl residue 475 and loss of heme.Mol Pharmacol. 2011 Nov;80(5):839-47. doi: 10.1124/mol.111.073783. Epub 2011 Aug 23. Mol Pharmacol. 2011. PMID: 21862689 Free PMC article.
-
Thr302 is the site for the covalent modification of human cytochrome P450 2B6 leading to mechanism-based inactivation by tert-butylphenylacetylene.Drug Metab Dispos. 2011 Dec;39(12):2431-9. doi: 10.1124/dmd.111.042176. Epub 2011 Sep 19. Drug Metab Dispos. 2011. PMID: 21930824 Free PMC article.
-
Targeting of the highly conserved threonine 302 residue of cytochromes P450 2B family during mechanism-based inactivation by aryl acetylenes.Arch Biochem Biophys. 2011 Mar 1;507(1):135-43. doi: 10.1016/j.abb.2010.09.006. Epub 2010 Sep 15. Arch Biochem Biophys. 2011. PMID: 20836985 Free PMC article. Review.
-
Structure and function of cytochromes P450 2B: from mechanism-based inactivators to X-ray crystal structures and back.Drug Metab Dispos. 2011 Jul;39(7):1113-21. doi: 10.1124/dmd.111.039719. Epub 2011 Apr 18. Drug Metab Dispos. 2011. PMID: 21502194 Free PMC article.
-
Structural analysis of mammalian cytochrome P450 2B4 covalently bound to the mechanism-based inactivator tert-butylphenylacetylene: insight into partial enzymatic activity.Biochemistry. 2011 Jun 7;50(22):4903-11. doi: 10.1021/bi200482g. Epub 2011 May 13. Biochemistry. 2011. PMID: 21510666 Free PMC article.
References
-
- Baillie TA, Davis MR. (1993) Mass spectrometry in the analysis of glutathione conjugates. Biol Mass Spectrom 22:319–325 - PubMed
-
- Blobaum AL, Kent UM, Alworth WL, Hollenberg PF. (2002) Mechanism-based inactivation of cytochromes P450 2E1 and 2E1 T303A by tert-butyl acetylenes: characterization of reactive intermediate adducts to the heme and apoprotein. Chem Res Toxicol 15:1561–1571 - PubMed
-
- CaJacob CA, Chan WK, Shephard E, Ortiz de Montellano PR. (1988) The catalytic site of rat hepatic lauric acid ω-hydroxylase: protein versus prosthetic heme alkylation in the ω-hydroxylation of acetylenic fatty acids. J Biol Chem 263:18640–18649 - PubMed
-
- Chan WK, Sui Z, Ortiz de Montellano PR. (1993) Determinants of protein modification versus heme alkylation: inactivation of cytochrome P450 1A1 by 1-ethynylpyrene and phenylacetylene. Chem Res Toxicol 6:38–45 - PubMed
-
- Domanski TL, Halpert JR. (2001) Analysis of mammalian cytochrome P450 structure and function by site-directed mutagenesis. Curr Drug Metab 2:117–137 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

