HIV-1 gp120-induced migration of dendritic cells is regulated by a novel kinase cascade involving Pyk2, p38 MAP kinase, and LSP1
- PMID: 19700666
- PMCID: PMC2766677
- DOI: 10.1182/blood-2009-02-206342
HIV-1 gp120-induced migration of dendritic cells is regulated by a novel kinase cascade involving Pyk2, p38 MAP kinase, and LSP1
Abstract
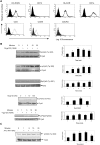
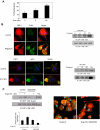
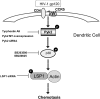
Targeting dendritic cell (DC) functions such as migration is a pivotal mechanism used by HIV-1 to disseminate within the host. The HIV-1 envelope protein is the most important of the virally encoded proteins that exploits the migratory capacity of DCs. In the present study, we elucidated the signaling machinery involved in migration of immature DCs (iDCs) in response to HIV-1 envelope protein. We observed that M-tropic HIV-1 glycoprotein 120 (gp120) induces phosphorylation of the nonreceptor tyrosine kinase, Pyk2. Inhibition of Pyk2 activity using a pharmacologic inhibitor, kinase-inactive Pyk2 mutant, and Pyk2-specific small interfering RNA blocked gp120-induced chemotaxis, confirming the role of Pyk2 in iDC migration. In addition, we also illustrated the importance of Pyk2 in iDC migration induced by virion-associated envelope protein, using aldithriol-2-inactivated M-tropic HIV-1 virus. Further analysis of the downstream signaling mechanisms involved in gp120-induced migration revealed that Pyk2 activates p38 mitogen-activated protein kinase, which in turn activates the F-actin-binding protein, leukocyte-specific protein 1, and enhances its association with actin. Taken together, our studies provide an insight into a novel gp120-mediated pathway that regulates DC chemotaxis and contributes to the dissemination of HIV-1 within an infected person.
Figures
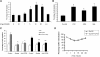



Similar articles
-
Slit2N/Robo1 inhibit HIV-gp120-induced migration and podosome formation in immature dendritic cells by sequestering LSP1 and WASp.PLoS One. 2012;7(10):e48854. doi: 10.1371/journal.pone.0048854. Epub 2012 Oct 31. PLoS One. 2012. PMID: 23119100 Free PMC article.
-
LPS-induced MCP-1 expression in human microvascular endothelial cells is mediated by the tyrosine kinase, Pyk2 via the p38 MAPK/NF-kappaB-dependent pathway.Mol Immunol. 2009 Feb;46(5):962-8. doi: 10.1016/j.molimm.2008.09.022. Epub 2008 Oct 26. Mol Immunol. 2009. PMID: 18954908 Free PMC article.
-
HIV-1 gp120 and chemokine activation of Pyk2 and mitogen-activated protein kinases in primary macrophages mediated by calcium-dependent, pertussis toxin-insensitive chemokine receptor signaling.Blood. 2001 Nov 15;98(10):2909-16. doi: 10.1182/blood.v98.10.2909. Blood. 2001. PMID: 11698270
-
Macrophage activation through CCR5- and CXCR4-mediated gp120-elicited signaling pathways.J Leukoc Biol. 2003 Nov;74(5):676-82. doi: 10.1189/jlb.0503206. Epub 2003 Jul 22. J Leukoc Biol. 2003. PMID: 12960231 Review.
-
PYK2, a hub of signaling networks in breast cancer progression.Trends Cell Biol. 2024 Apr;34(4):312-326. doi: 10.1016/j.tcb.2023.07.006. Epub 2023 Aug 15. Trends Cell Biol. 2024. PMID: 37586982 Review.
Cited by
-
Initial HIV mucosal infection and dendritic cells.EMBO Mol Med. 2013 May;5(5):658-60. doi: 10.1002/emmm.201202763. EMBO Mol Med. 2013. PMID: 23653303 Free PMC article. No abstract available.
-
LSP1-myosin1e bimolecular complex regulates focal adhesion dynamics and cell migration.FASEB J. 2021 Feb;35(2):e21268. doi: 10.1096/fj.202000740RR. FASEB J. 2021. PMID: 33470457 Free PMC article.
-
Sphingomyelin synthase 2, but not sphingomyelin synthase 1, is involved in HIV-1 envelope-mediated membrane fusion.J Biol Chem. 2014 Oct 31;289(44):30842-30856. doi: 10.1074/jbc.M114.574285. Epub 2014 Sep 17. J Biol Chem. 2014. PMID: 25231990 Free PMC article.
-
All-Round Manipulation of the Actin Cytoskeleton by HIV.Viruses. 2018 Feb 5;10(2):63. doi: 10.3390/v10020063. Viruses. 2018. PMID: 29401736 Free PMC article. Review.
-
Two macrophages, osteoclasts and microglia: from development to pleiotropy.Bone Res. 2021 Feb 10;9(1):11. doi: 10.1038/s41413-020-00134-w. Bone Res. 2021. PMID: 33568650 Free PMC article. Review.
References
-
- Chougnet C, Gessani S. Role of gp120 in dendritic cell dysfunction in HIV infection. J Leukoc Biol. 2006;80(5):994–1000. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

