Intestinal glucose uptake protects liver from lipopolysaccharide and D-galactosamine, acetaminophen, and alpha-amanitin in mice
- PMID: 19700751
- PMCID: PMC2731125
- DOI: 10.2353/ajpath.2009.090071
Intestinal glucose uptake protects liver from lipopolysaccharide and D-galactosamine, acetaminophen, and alpha-amanitin in mice
Abstract
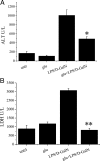
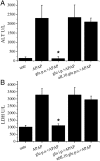
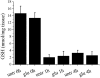
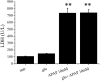
We have recently observed that oral administration of D-glucose saves animals from lipopolysaccharide (LPS)-induced death. This effect is the likely consequence of glucose-induced activation of the sodium-dependent glucose transporter-1. In this study, we investigated possible hepatoprotective effects of glucose-induced, sodium-dependent, glucose transporter-1 activation. We show that oral administration of D-glucose, but not of either D-fructose or sucrose, prevents LPS-induced liver injury, as well as liver injury and death induced by an overdose of acetaminophen. In both of these models, physiological liver morphology is maintained and organ protection is confirmed by unchanged levels of the circulating markers of hepatotoxicity, such as alanine transaminase or lactate dehydrogenase. In addition, D-glucose was found to protect the liver from alpha-amanitin-induced liver injury. In this case, in contrast to the previously described models, a second signal had to be present in addition to glucose to achieve protective efficacy. Toll-like receptor 4 stimulation that was induced by low doses of LPS was identified as such a second signal. Eventually, the protective effect of orally administered glucose on liver injury induced by LPS, overdose of acetaminophen, or alpha-amanitin was shown to be mediated by the anti-inflammatory cytokine interleukin-10. These findings, showing glucose-induced protective effects in several animal models of liver injury, might be relevant in view of possible therapeutic interventions against different forms of acute hepatic injury.
Figures




References
-
- Schiodt FV, Antillasoy E, Shakil AO, Schiff ER, Caldwell C, Kowdley KV, Stibling R, Crippin JS, Flamm S, Somberg KA, Rosen H, McCashland TM, Hay JE, Lee WM. Etiology and outcome for 295 patients with acute liver failure in the United States. Liver Transpl Surg. 1999;5:29–34. - PubMed
-
- Dass M, Plevak DJ, Wijdicks EF, Rakela J, Wiesner RH, Piepgras DG, Dunn WF, Steers JL. Acute liver failure: results of a 5-year clinical protocol. Liver Transpl Surg. 1995;1:210–219. - PubMed
-
- Luedde T, Trautwein C. Intracellular survival pathways in the liver. Liver Int. 2006;26:1163–1174. - PubMed
-
- Palazzo M, Gariboldi S, Zanobbio L, Selleri S, Dusio GF, Mauro V, Rossini A, Balsari A, Rumio C. SGLT-1 as a novel immunological player in the intestinal mucosa. J Immunol. 2008;181:3126–3136. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

