Overexpression of MHC class I heavy chain protein in young skeletal muscle leads to severe myositis: implications for juvenile myositis
- PMID: 19700752
- PMCID: PMC2731122
- DOI: 10.2353/ajpath.2009.090196
Overexpression of MHC class I heavy chain protein in young skeletal muscle leads to severe myositis: implications for juvenile myositis
Abstract
Folding and transport of proteins, such as major histocompatibility complex (MHC) class I, through the endoplasmic reticulum (ER) is tightly regulated in all cells, including muscle tissue, where the specialized ER sarcoplasmic reticulum is also critical to muscle fiber function. Overexpression of MHC class I protein is a common feature of many muscle pathologies including idiopathic myositis and can induce ER stress. However, there has been no comparison of the consequences of MHC overexpression in muscle at different ages. We have adapted a transgenic model of myositis induced by overexpression of MHC class I protein in skeletal muscle to investigate the effects of this protein overload on young muscle fibers, as compared with adult tissue. We find a markedly more severe disease phenotype in young mice, with rapid onset of muscle weakness and pathology. Gene expression profiling to compare the two models indicates rapid onset of ER stress in young muscle tissue but also that gene expression of key muscle structural proteins is affected more rapidly in young mice than adults after this insult. This novel model has important implications for our understanding of muscle pathology in dermatomyositis of both adults and children.
Figures



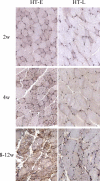
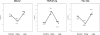
References
-
- Rider LG, Miller FW. Classification and treatment of the juvenile idiopathic inflammatory myopathies. Rheum Dis Clin North Am. 1997;23:619–655. - PubMed
-
- Wedderburn LR, Li CK. Paediatric idiopathic inflammatory muscle disease. Best Pract Res Clin Rheumatol. 2004;18:345–358. - PubMed
-
- Huber AM, Lang B, LeBlanc CM, Birdi N, Bolaria RK, Malleson P, MacNeil I, Momy JA, Avery G, Feldman BM. Medium- and long-term functional outcomes in a multicenter cohort of children with juvenile dermatomyositis. Arthritis Rheum. 2000;43:541–549. - PubMed
-
- McCann LJ, Juggins AD, Maillard SM, Wedderburn LR, Davidson JE, Murray KJ, Pilkington CA. The Juvenile Dermatomyositis National Registry and Repository (UK and Ireland)–clinical characteristics of children recruited within the first 5 years. Rheumatology. 2006;45:1255–1260. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

