A key role for the integrin alpha2beta1 in experimental and developmental angiogenesis
- PMID: 19700757
- PMCID: PMC2731151
- DOI: 10.2353/ajpath.2009.090234
A key role for the integrin alpha2beta1 in experimental and developmental angiogenesis
Abstract
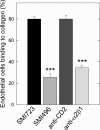
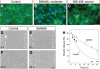
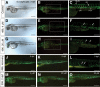
The alpha2beta1 integrin receptor plays a key role in angiogenesis. Here we investigated the effects of small molecule inhibitors (SMIs) designed to disrupt integrin alpha2 I or beta1 I-like domain function on angiogenesis. In unchallenged endothelial cells, fibrillar collagen induced robust capillary morphogenesis. In contrast, tube formation was significantly reduced by SMI496, a beta1 I-like domain inhibitor and by function-blocking anti-alpha2beta1 but not -alpha1beta1 antibodies. Endothelial cells bound fluorescein-labeled collagen I fibrils, an interaction specifically inhibited by SMI496. Moreover, SMI496 caused cell retraction and cytoskeletal collapse of endothelial cells as well as delayed endothelial cell wound healing. SMI activities were examined in vivo by supplementing the growth medium of zebrafish embryos expressing green fluorescent protein under the control of the vascular endothelial growth factor receptor-2 promoter. SMI496, but not a control compound, interfered with angiogenesis in vivo by reversibly inhibiting sprouting from the axial vessels. We further characterized zebrafish alpha2 integrin and discovered that this integrin is highly conserved, especially the I domain. Notably, a similar vascular phenotype was induced by morpholino-mediated knockdown of the integrin alpha2 subunit. By live videomicroscopy, we confirmed that the vessels were largely nonfunctional in the absence of alpha2beta1 integrin. Collectively, our results provide strong biochemical and genetic evidence of a central role for alpha2beta1 integrin in experimental and developmental angiogenesis.
Figures
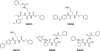




Similar articles
-
Angiogenesis promoted by vascular endothelial growth factor: regulation through alpha1beta1 and alpha2beta1 integrins.Proc Natl Acad Sci U S A. 1997 Dec 9;94(25):13612-7. doi: 10.1073/pnas.94.25.13612. Proc Natl Acad Sci U S A. 1997. PMID: 9391074 Free PMC article.
-
Functional overlap and cooperativity among alphav and beta1 integrin subfamilies during skin angiogenesis.J Invest Dermatol. 2003 Jun;120(6):1100-9. doi: 10.1046/j.1523-1747.2003.12236.x. J Invest Dermatol. 2003. PMID: 12787141
-
Aggretin Venom Polypeptide as a Novel Anti-angiogenesis Agent by Targeting Integrin alpha2beta1.Sci Rep. 2017 Mar 2;7:43612. doi: 10.1038/srep43612. Sci Rep. 2017. PMID: 28252668 Free PMC article.
-
Alternagin-C, a disintegrin-like protein from the venom of Bothrops alternatus, modulates alpha2beta1 integrin-mediated cell adhesion, migration and proliferation.Braz J Med Biol Res. 2005 Oct;38(10):1505-11. doi: 10.1590/s0100-879x2005001000007. Epub 2005 Sep 6. Braz J Med Biol Res. 2005. PMID: 16172743 Review.
-
α2β1 Integrin.Adv Exp Med Biol. 2014;819:41-60. doi: 10.1007/978-94-017-9153-3_3. Adv Exp Med Biol. 2014. PMID: 25023166 Review.
Cited by
-
The integrin coactivator kindlin-2 plays a critical role in angiogenesis in mice and zebrafish.Blood. 2011 May 5;117(18):4978-87. doi: 10.1182/blood-2010-11-321182. Epub 2011 Mar 4. Blood. 2011. PMID: 21378273 Free PMC article.
-
Molecular basis for pericyte-induced capillary tube network assembly and maturation.Front Cell Dev Biol. 2022 Aug 22;10:943533. doi: 10.3389/fcell.2022.943533. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36072343 Free PMC article. Review.
-
Proteoglycan form and function: A comprehensive nomenclature of proteoglycans.Matrix Biol. 2015 Mar;42:11-55. doi: 10.1016/j.matbio.2015.02.003. Epub 2015 Feb 18. Matrix Biol. 2015. PMID: 25701227 Free PMC article. Review.
-
Conditional expression of endorepellin in the tumor vasculature attenuates breast cancer growth, angiogenesis and hyaluronan deposition.Matrix Biol. 2023 Apr;118:92-109. doi: 10.1016/j.matbio.2023.03.005. Epub 2023 Mar 11. Matrix Biol. 2023. PMID: 36907428 Free PMC article.
-
Role of tyrosine phosphatase SHP-1 in the mechanism of endorepellin angiostatic activity.Blood. 2009 Nov 26;114(23):4897-906. doi: 10.1182/blood-2009-02-207134. Epub 2009 Sep 29. Blood. 2009. PMID: 19789387 Free PMC article.
References
-
- Folkman J. Role of angiogenesis in tumor growth and metastasis. Semin Oncol. 2002;29:15–18. - PubMed
-
- Davis GE, Senger DR. Endothelial extracellular matrix: biosynthesis, remodeling, and functions during vascular morphogenesis and neovessel stabilization. Circ Res. 2005;97:1093–1107. - PubMed
-
- Davis GE, Senger DR. Extracellular matrix mediates a molecular balance between vascular morphogenesis and regression. Curr Opin Hematol. 2008;15:197–203. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

