Headspace, volatile and semi-volatile organic compounds diversity and radical scavenging activity of ultrasonic solvent extracts from Amorpha fruticosa honey samples
- PMID: 19701118
- PMCID: PMC6254909
- DOI: 10.3390/molecules14082717
Headspace, volatile and semi-volatile organic compounds diversity and radical scavenging activity of ultrasonic solvent extracts from Amorpha fruticosa honey samples
Abstract
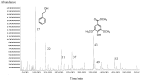
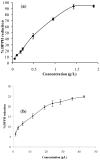
Volatile organic compounds of Amorpha fruticosa honey samples were isolated by headspace solid-phase microextraction (HS-SPME) and ultrasonic solvent extraction (USE), followed by gas chromatography and mass spectrometry analyses (GC, GC-MS), in order to obtain complementary data for overall characterization of the honey aroma. The headspace of the honey was dominated by 2-phenylethanol (38.3-58.4%), while other major compounds were trans- and cis-linalool oxides, benzaldehyde and benzyl alcohol. 2-Phenylethanol (10.5-16.8%) and methyl syringate (5.8-8.2%) were the major compounds of ultrasonic solvent extracts, with an array of small percentages of linalool, benzene and benzoic acid derivatives, aliphatic hydrocarbons and alcohols, furan derivatives and others. The scavenging ability of the series of concentrations of the honey ultrasonic solvent extracts and the corresponding honey samples was tested by a DPPH (1,1-diphenyl-2-picrylhydrazyl) assay. Approximately 25 times lower concentration ranges (up to 2 g/L) of the extracts exhibited significantly higher free radical scavenging potential with respect to the honey samples.
Figures

Similar articles
-
Volatiles from a rare Acer spp. honey sample from Croatia.Molecules. 2010 Jun 24;15(7):4572-82. doi: 10.3390/molecules15074572. Molecules. 2010. PMID: 20657377 Free PMC article.
-
Bioorganic diversity of rare Coriandrum sativum L. honey: unusual chromatographic profiles containing derivatives of linalool/oxygenated methoxybenzene.Chem Biodivers. 2013 Aug;10(8):1549-58. doi: 10.1002/cbdv.201300074. Chem Biodivers. 2013. PMID: 23939803
-
The volatile profiles of a rare apple (Malus domestica Borkh.) honey: shikimic acid-pathway derivatives, terpenes, and others.Chem Biodivers. 2013 Sep;10(9):1638-52. doi: 10.1002/cbdv.201200404. Chem Biodivers. 2013. PMID: 24078598
-
Applications of solid-phase microextraction and gas chromatography/mass spectrometry (SPME-GC/MS) in the study of grape and wine volatile compounds.Molecules. 2014 Dec 18;19(12):21291-309. doi: 10.3390/molecules191221291. Molecules. 2014. PMID: 25529017 Free PMC article. Review.
-
[Recent advances in the application of headspace gas chromatography-mass spectrometry].Se Pu. 2018 Oct 8;36(10):962-971. doi: 10.3724/SP.J.1123.2018.05013. Se Pu. 2018. PMID: 30378354 Review. Chinese.
Cited by
-
Bioactivity and Chemical Characterization of Sudanese Bee Honey: Crude Acacia and Its Organic Extracts.Biomed Res Int. 2022 Aug 17;2022:8441239. doi: 10.1155/2022/8441239. eCollection 2022. Biomed Res Int. 2022. PMID: 36033555 Free PMC article.
-
Analysis of volatile compounds of Malaysian Tualang (Koompassia excelsa) honey using gas chromatography mass spectrometry.Afr J Tradit Complement Altern Med. 2012 Dec 31;10(2):180-8. doi: 10.4314/ajtcam.v10i2.2. eCollection 2013. Afr J Tradit Complement Altern Med. 2012. PMID: 24146441 Free PMC article.
-
Screening of Satureja subspicata Vis. Honey by HPLC-DAD, GC-FID/MS and UV/VIS: Prephenate Derivatives as Biomarkers.Molecules. 2016 Mar 21;21(3):377. doi: 10.3390/molecules21030377. Molecules. 2016. PMID: 27007367 Free PMC article.
-
Quantifying the sweetness intensity and impact of aroma in honey from four floral sources.J Food Sci. 2024 Dec;89(12):9732-9741. doi: 10.1111/1750-3841.17461. Epub 2024 Oct 22. J Food Sci. 2024. PMID: 39437307 Free PMC article.
-
Oak (Quercus frainetto Ten.) honeydew honey--approach to screening of volatile organic composition and antioxidant capacity (DPPH and FRAP assay).Molecules. 2010 May 25;15(5):3744-56. doi: 10.3390/molecules15053744. Molecules. 2010. PMID: 20657511 Free PMC article.
References
-
- Bertelli D., Papotti G., Lolli M., Sabatini A.G., Plessi M. Development of an HS-SPME-GC method to determine the methyl anthranilate in Citrus honeys. Food Chem. 2008;108:297–303. doi: 10.1016/j.foodchem.2007.10.019. - DOI
-
- de la Fuente E., Valencia-Barrera R.M., Martïnez-Castro I., Sanz J. Occurrence of 2-hydroxy-5-methyl-3-hexanone and 3-hydroxy-5-methyl-2-hexanone as indicators of botanic origin in eucalyptus honeys. Food Chem. 2007;103:1176–1180. doi: 10.1016/j.foodchem.2006.10.020. - DOI
-
- Castro-Vázquez, Díaz-Maroto M.C., González-Viñas M.A., Pérez-Coello M.S. Differentiation of monofloral citrus, rosemary, eucalyptus, lavender, thyme and heather honeys based on volatile composition and sensory descriptive analysis. Food Chem. 2009;112:1022–1030. doi: 10.1016/j.foodchem.2008.06.036. - DOI
-
- Guyot C., Scheirman V., Collin S. Floral origin markers of heather honeys: Calluna vulgaris and Erica arborea. Food Chem. 1999;64:3–11. doi: 10.1016/S0308-8146(98)00122-8. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

