Synthesis of heteroaromatic compounds by oxidative aromatization using an activated carbon/molecular oxygen system
- PMID: 19701145
- PMCID: PMC6254805
- DOI: 10.3390/molecules14083073
Synthesis of heteroaromatic compounds by oxidative aromatization using an activated carbon/molecular oxygen system
Abstract
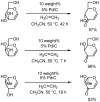
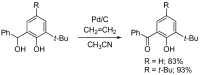
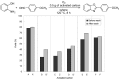
A variety of heteroaromatic compounds, such as substituted pyridines, pyrazoles, indoles, 2-substituted imidazoles, 2-substituted imidazoles, 2-arylbenzazoles and pyrimidin-2(1H)-ones are synthesized by oxidative aromatization using the activated carbon and molecular oxygen system. Mechanistic study focused on the role of activated carbon in the synthesis of 2-arylbenzazoles is also discussed. In the final section, we will disclose the efficient synthesis of substituted 9,10-anthracenes via oxidative aromatization.
Figures
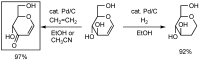


References
-
- Hudlicky M. Oxidations in Organic Chemistry. American Chemical Society; Washington, DC, USA: 1990. ACS Monograph Series.
-
- Hayashi M., Yamada K., Arikita O. Hydrogen Transfer of D-Glycals by Palladium Complexes under Ethylene Atmosphere. Tetrahedron Lett. 1999;40:1171–1174. doi: 10.1016/S0040-4039(98)02556-8. - DOI
-
- Hayashi M., Yamada K., Arikita O. Practical and Catalytic Synthesis of 1,5-Anhydrohex-1-en-3-uloses. Tetrahedron. 1999;55:8331–8340.
-
- Hayashi M., Yamada K., Nakayama S. Catalytic Hydrogen Transfer Reaction of Benzylic and Allylic Alcohols with Palladium Compound in the Presence of Vinyl Acetate or under Ethylene Atmosphere. J. Chem. Soc., Perkin Trans.1. 2000:1501–1503. doi: 10.1039/b001385o. - DOI
-
- Hayashi M., Yamada K., Nakayama S., Hayashi H., Yamazaki S. Environmentally Benign Oxidation Method Using Novel Palladium Catalyst System. Green Chem. 2000:257–260.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

