Arrested maturation of excitatory synapses in autosomal dominant lateral temporal lobe epilepsy
- PMID: 19701204
- PMCID: PMC2759408
- DOI: 10.1038/nm.2019
Arrested maturation of excitatory synapses in autosomal dominant lateral temporal lobe epilepsy
Abstract
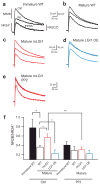
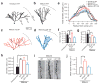
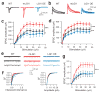
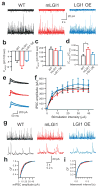
A subset of central glutamatergic synapses are coordinately pruned and matured by unresolved mechanisms during postnatal development. We report that the human epilepsy gene LGI1, encoding leucine-rich, glioma-inactivated protein-1 and mutated in autosomal dominant lateral temporal lobe epilepsy (ADLTE), mediates this process in hippocampus. We created transgenic mice either expressing a truncated mutant LGI1 (835delC) found in ADLTE or overexpressing a wild-type LGI1. We discovered that the normal postnatal maturation of presynaptic and postsynaptic functions was arrested by the 835delC mutant LGI1, and contrastingly, was magnified by excess wild-type LGI1. Concurrently, mutant LGI1 inhibited dendritic pruning and increased the spine density to markedly increase excitatory synaptic transmission. Inhibitory transmission, by contrast, was unaffected. Furthermore, mutant LGI1 promoted epileptiform discharge in vitro and kindling epileptogenesis in vivo with partial gamma-aminobutyric acid(A) (GABA(A)) receptor blockade. Thus, LGI1 represents a human gene mutated to promote epilepsy through impaired postnatal development of glutamatergic circuits.
Figures

Comment in
-
Epilepsy: synapses stuck in childhood.Nat Med. 2009 Oct;15(10):1126-7. doi: 10.1038/nm1009-1126. Nat Med. 2009. PMID: 19812567 No abstract available.
References
-
- Waites CL, Craig AM, Garner CC. Mechanisms of vertebrate synaptogenesis. Annu Rev Neurosci. 2005;28:251–274. - PubMed
-
- Chen C, Regehr WG. Developmental remodeling of the retinogeniculate synapse. Neuron. 2000;28:955–966. - PubMed
-
- Rihn LL, Claiborne BJ. Dendritic growth and regression in rat dentate granule cells during late postnatal development. Brain Res Dev Brain Res. 1990;54:115–124. - PubMed
-
- Bolshakov VY, Siegelbaum SA. Regulation of hippocampal transmitter release during development and long-term potentiation. Science. 1995;269:1730–1734. - PubMed
-
- Chavis P, Westbrook G. Integrins mediate functional pre- and postsynaptic maturation at a hippocampal synapse. Nature. 2001;411:317–321. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

