n-Butylidenephthalide induced apoptosis in the A549 human lung adenocarcinoma cell line by coupled down-regulation of AP-2alpha and telomerase activity
- PMID: 19701232
- PMCID: PMC4007174
- DOI: 10.1038/aps.2009.124
n-Butylidenephthalide induced apoptosis in the A549 human lung adenocarcinoma cell line by coupled down-regulation of AP-2alpha and telomerase activity
Expression of concern in
-
Editorial Expression of Concern: n-Butylidenephthalide induced apoptosis in the A549 human lung adenocarcinoma cell line by coupled down-regulation of AP-2α and telomerase activity.Acta Pharmacol Sin. 2026 Jan;47(1):272. doi: 10.1038/s41401-025-01660-1. Acta Pharmacol Sin. 2026. PMID: 40983653 Free PMC article. No abstract available.
Abstract
Aim: To investigate the role of hTERT gene expression and AP-2alpha in n-butylidenephthalide (n-BP)-induced apoptosis in A549 lung cancer cells.
Methods: Viability of A549 cells was measured by MTT assay. Protein expression was determined by Western blot. Telomerase activity was measured using the modified telomere repeat amplification protocol (TRAP) assay. Xenograft mice were used as a model system to study the cytotoxic effect of n-BP in vivo. The morphology of tumor was examined by immunohistochemical staining.
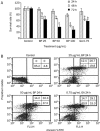
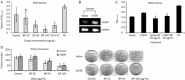
Results: The growth of A549 lung cancer cells treated with n-BP was significantly inhibited. Telomerase activity and hTERT mRNA expression were determined by telomeric repeat amplification protocol and reverse transcription-polymerase chain reaction, respectively. n-BP inhibited telomerase activity and hTERT mRNA expression in A549 cells while overexpression of hTERT could abolish BP-induced growth inhibition in the A549 cells. We also showed that hTERT promoter activity in the presence of n-BP was mediated via AP-2alpha. We saw an inhibition of tumor growth when nude mice carrying A549 subcutaneous xenograft tumors were treated with n-BP. Immunohistochemistry of this tumor tissue also showed a decrease in the expression of hTERT.
Conclusion: The antiproliferative effects of n-BP on A549 cells in vitro and in vivo suggest a novel clinical application of this compound in the treatment of lung cancers.
Figures




References
-
- Blackburn EH. Telomerases. Annu Rev Biochem. 1992;61:113–29. - PubMed
-
- Greider CW, Blackburn EH. A telomeric sequence in the RNA of tetrahymena telomerase required for telomere repeat synthesis. Nature. 1989;337:331–7. - PubMed
-
- Blackburn EH. Structure and function of telomeres. Nature. 1991;350:569–73. - PubMed
-
- Feng J, Funk WD, Wang SS, Weinrich SL, Avilion AA, Chiu CP, et al. The RNA component of human telomerase. Science. 1995;269:1236–41. - PubMed
-
- Nakamura TM, Morin GB, Chapman KB, Weinrich SL, Andrews WH, Lingner J, et al. Telomerase catalytic subunit homologs from fission yeast and human. Science. 1997;277:955–9. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

