Dithiolethione compounds inhibit Akt signaling in human breast and lung cancer cells by increasing PP2A activity
- PMID: 19701246
- PMCID: PMC3472634
- DOI: 10.1038/onc.2009.244
Dithiolethione compounds inhibit Akt signaling in human breast and lung cancer cells by increasing PP2A activity
Abstract
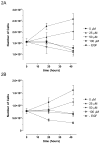
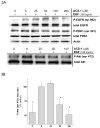
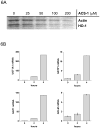
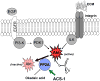
The chemopreventative effects of dithiolethione compounds are attributed to their activation of antioxidant response elements (AREs) by reacting with the Nrf2/Keap1 protein complex. In this study, we show antiproliferative effects of the dithiolethione compound ACS-1 in human cancer cell lines (A549 and MDA-MB-231) by increasing the activity of the tumor suppressor protein phoshatase 2A (PP2A). ACS-1 inhibited epidermal growth factor (EGF)-induced cellular proliferation in a concentration- and time-dependent manner. Akt activation, as determined by serine-473 phosphorylation, was inhibited by ACS-1 in cells stimulated with either EGF or fibronectin. Furthermore, ACS-1 inhibited mammalian target of rapamycin signaling and decreased c-myc protein levels. ACS-1 did not proximally alter EGF receptor or integrin signaling, but caused a concentration-dependent increase in PP2A activity. The effect of ACS-1 on Akt activation was not observed in the presence of the PP2A inhibitor okadaic acid. ACS-1 effects on PP2A activity were independent of ARE activation and cAMP formation. In addition to ACS-1, other dithiolethione compounds showed similar effects in reducing Akt activation, suggesting that this class of compounds may have other effects beyond chemoprevention.
Conflict of interest statement
Conflict of interest
Piero Del Soldato and Anna Sparatore are shareholders of Sulfidris, Milan, Italy. This company has patent rights on some reagents used in this study.
Figures








References
-
- Andjelkovic M, Jakubowicz T, Cron P, Ming XF, Han JW, Hemmings BA. Activation and phosphorylation of a pleckstrin homology domain containing protein kinase (RAC-PK/PKB) promoted by serum and protein phosphatase inhibitors. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:5699–5704. - PMC - PubMed
-
- Bae EJ, Yang Yoon Mee, Kim Jin Wan, Kim Sang Geon. Identification of a novel class of dithiolethiones that prevent hepatic insulin resistance via the adenosine monophosphate-activated protein kinase-p70 ribosomal S6 kinase-1 pathway. Hepatology. 2007;46:730–739. - PubMed
-
- Busserolles J, Megías J, Terencio MC, Alcaraz MJ. Heme oxygenase-1 inhibits apoptosis in Caco-2 cells via activation of Akt pathway. The International Journal of Biochemistry & Cell Biology. 2006;38:1510–1517. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

