Highly efficient differentiation and enrichment of spinal motor neurons derived from human and monkey embryonic stem cells
- PMID: 19701462
- PMCID: PMC2726947
- DOI: 10.1371/journal.pone.0006722
Highly efficient differentiation and enrichment of spinal motor neurons derived from human and monkey embryonic stem cells
Abstract
Background: There are no cures or efficacious treatments for severe motor neuron diseases. It is extremely difficult to obtain naïve spinal motor neurons (sMNs) from human tissues for research due to both technical and ethical reasons. Human embryonic stem cells (hESCs) are alternative sources. Several methods for MN differentiation have been reported. However, efficient production of naïve sMNs and culture cost were not taken into consideration in most of the methods.
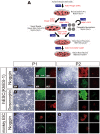
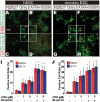
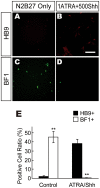
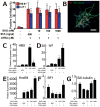
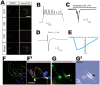
Methods/principal findings: We aimed to establish protocols for efficient production and enrichment of sMNs derived from pluripotent stem cells. Nestin+ neural stem cell (NSC) clusters were induced by Noggin or a small molecule inhibitor of BMP signaling. After dissociation of NSC clusters, neurospheres were formed in a floating culture containing FGF2. The number of NSCs in neurospheres could be expanded more than 30-fold via several passages. More than 33% of HB9+ sMN progenitor cells were observed after differentiation of dissociated neurospheres by all-trans retinoic acid (ATRA) and a Shh agonist for another week on monolayer culture. HB9+ sMN progenitor cells were enriched by gradient centrifugation up to 80% purity. These HB9+ cells differentiated into electrophysiologically functional cells and formed synapses with myotubes during a few weeks after ATRA/SAG treatment.
Conclusions and significance: The series of procedures we established here, namely neural induction, NSC expansion, sMN differentiation and sMN purification, can provide large quantities of naïve sMNs derived from human and monkey pluripotent stem cells. Using small molecule reagents, reduction of culture cost could be achieved.
Conflict of interest statement
Figures
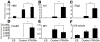

Similar articles
-
Rapid, efficient, and simple motor neuron differentiation from human pluripotent stem cells.Mol Brain. 2015 Dec 1;8(1):79. doi: 10.1186/s13041-015-0172-4. Mol Brain. 2015. PMID: 26626025 Free PMC article.
-
Efficient differentiation of mouse embryonic stem cells into motor neurons.J Vis Exp. 2012 Jun 9;(64):e3813. doi: 10.3791/3813. J Vis Exp. 2012. PMID: 22711008 Free PMC article.
-
Derivation of Motor Neurons from three Clonal Human Embryonic Stem Cell Lines.Curr Neurovasc Res. 2006 Nov;3(4):281-8. doi: 10.2174/156720206778792902. Curr Neurovasc Res. 2006. PMID: 17109623
-
Motor neuron derivation from human embryonic and induced pluripotent stem cells: experimental approaches and clinical perspectives.Stem Cell Res Ther. 2014 Jul 14;5(4):87. doi: 10.1186/scrt476. Stem Cell Res Ther. 2014. PMID: 25157556 Free PMC article. Review.
-
Therapeutic potential of motor neurons differentiated from embryonic stem cells and induced pluripotent stem cells.Arch Med Res. 2012 Jan;43(1):1-10. doi: 10.1016/j.arcmed.2012.01.007. Epub 2012 Jan 30. Arch Med Res. 2012. PMID: 22293229 Review.
Cited by
-
Substantial Differentiation of Human Neural Stem Cells Into Motor Neurons on a Biomimetic Polyurea.Macromol Biosci. 2015 Sep;15(9):1206-11. doi: 10.1002/mabi.201500066. Epub 2015 May 29. Macromol Biosci. 2015. PMID: 26033933 Free PMC article.
-
Utility of Induced Pluripotent Stem Cells for the Study and Treatment of Genetic Diseases: Focus on Childhood Neurological Disorders.Front Mol Neurosci. 2016 Sep 6;9:78. doi: 10.3389/fnmol.2016.00078. eCollection 2016. Front Mol Neurosci. 2016. PMID: 27656126 Free PMC article. Review.
-
Efficient and rapid conversion of human astrocytes and ALS mouse model spinal cord astrocytes into motor neuron-like cells by defined small molecules.Mil Med Res. 2020 Sep 6;7(1):42. doi: 10.1186/s40779-020-00271-7. Mil Med Res. 2020. PMID: 32892745 Free PMC article.
-
Viability of primary cultured retinal neurons in a hyperglycemic condition.Neural Regen Res. 2013 Feb 15;8(5):410-9. doi: 10.3969/j.issn.1673-5374.2013.05.004. Neural Regen Res. 2013. PMID: 25206682 Free PMC article.
-
Transgenic enrichment of mouse embryonic stem cell-derived progenitor motor neurons.Stem Cell Res. 2012 May;8(3):368-78. doi: 10.1016/j.scr.2011.12.003. Epub 2011 Dec 13. Stem Cell Res. 2012. PMID: 22297157 Free PMC article.
References
-
- Briscoe J, Sussel L, Serup P, Hartigan-O'Connor D, Jessell TM, et al. Homeobox gene Nkx2.2 and specification of neuronal identity by graded Sonic hedgehog signalling. Nature. 1999;398:622–627. - PubMed
-
- Ericson J, Morton S, Kawakami A, Roelink H, Jessell TM. Two critical periods of Sonic Hedgehog signaling required for the specification of motor neuron identity. Cell. 1996;87:661–673. - PubMed
-
- Marti E, Takada R, Bumcrot DA, Sasaki H, McMahon AP. Distribution of Sonic hedgehog peptides in the developing chick and mouse embryo. Development. 1995;121:2537–2547. - PubMed
-
- Roelink H, Porter JA, Chiang C, Tanabe Y, Chang DT, et al. Floor plate and motor neuron induction by different concentrations of the amino-terminal cleavage product of sonic hedgehog autoproteolysis. Cell. 1995;81:445–455. - PubMed
-
- Tanabe Y, Roelink H, Jessell TM. Induction of motor neurons by Sonic hedgehog is independent of floor plate differentiation. Curr Biol. 1995;5:651–658. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

