A crucial role for TNF-alpha in mediating neutrophil influx induced by endogenously generated or exogenous chemokines, KC/CXCL1 and LIX/CXCL5
- PMID: 19702783
- PMCID: PMC2765597
- DOI: 10.1111/j.1476-5381.2009.00367.x
A crucial role for TNF-alpha in mediating neutrophil influx induced by endogenously generated or exogenous chemokines, KC/CXCL1 and LIX/CXCL5
Abstract
Background and purpose: Chemokines orchestrate neutrophil recruitment to inflammatory foci. In the present study, we evaluated the participation of three chemokines, KC/CXCL1, MIP-2/CXCL2 and LIX/CXCL5, which are ligands for chemokine receptor 2 (CXCR2), in mediating neutrophil recruitment in immune inflammation induced by antigen in immunized mice.
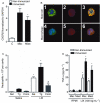
Experimental approach: Neutrophil recruitment was assessed in immunized mice challenged with methylated bovine serum albumin, KC/CXCL1, LIX/CXCL5 or tumour necrosis factor (TNF)-alpha. Cytokine and chemokine levels were determined in peritoneal exudates and in supernatants of macrophages and mast cells by elisa. CXCR2 and intercellular adhesion molecule 1 (ICAM-1) expression was determined using immunohistochemistry and confocal microscopy.
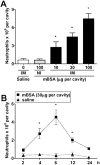
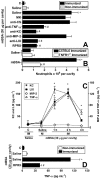
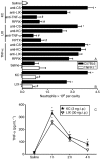
Key results: Antigen challenge induced dose- and time-dependent neutrophil recruitment and production of KC/CXCL1, LIX/CXCL5 and TNF-alpha, but not MIP-2/CXCL2, in peritoneal exudates. Neutrophil recruitment was inhibited by treatment with reparixin (CXCR1/2 antagonist), anti-KC/CXCL1, anti-LIX/CXCL5 or anti-TNF-alpha antibodies and in tumour necrosis factor receptor 1-deficient mice. Intraperitoneal injection of KC/CXCL1 and LIX/CXCL5 induced dose- and time-dependent neutrophil recruitment and TNF-alpha production, which were inhibited by reparixin or anti-TNF-alpha treatment. Macrophages and mast cells expressed CXCR2 receptors. Increased macrophage numbers enhanced, while cromolyn sodium (mast cell stabilizer) diminished, LIX/CXCL5-induced neutrophil recruitment. Macrophages and mast cells from immunized mice produced TNF-alpha upon LIX/CXCL5 stimulation. Methylated bovine serum albumin induced expression of ICAM-1 on mesenteric vascular endothelium, which was inhibited by anti-TNF-alpha or anti-LIX/CXCL5.
Conclusion and implications: Following antigen challenge, CXCR2 ligands are produced and act on macrophages and mast cells triggering the production of TNF-alpha, which synergistically contribute to neutrophil recruitment through induction of the expression of ICAM-1.
Figures


Similar articles
-
CXCL1/KC and CXCL5/LIX are selectively produced by corneal fibroblasts and mediate neutrophil infiltration to the corneal stroma in LPS keratitis.J Leukoc Biol. 2007 Mar;81(3):786-92. doi: 10.1189/jlb.0806502. Epub 2006 Nov 16. J Leukoc Biol. 2007. PMID: 17110418 Free PMC article.
-
CXCR2-specific chemokines mediate leukotriene B4-dependent recruitment of neutrophils to inflamed joints in mice with antigen-induced arthritis.Arthritis Rheum. 2008 Jul;58(7):2030-40. doi: 10.1002/art.23597. Arthritis Rheum. 2008. PMID: 18576322
-
The chemokine receptors CXCR1/CXCR2 modulate antigen-induced arthritis by regulating adhesion of neutrophils to the synovial microvasculature.Arthritis Rheum. 2008 Aug;58(8):2329-37. doi: 10.1002/art.23622. Arthritis Rheum. 2008. PMID: 18668539
-
Combined anti CXC receptors 1 and 2 therapy is a promising anti-inflammatory treatment for respiratory diseases by reducing neutrophil migration and activation.Pulm Pharmacol Ther. 2015 Oct;34:37-45. doi: 10.1016/j.pupt.2015.08.002. Epub 2015 Aug 10. Pulm Pharmacol Ther. 2015. PMID: 26271598 Review.
-
CXCR2 in acute lung injury.Mediators Inflamm. 2012;2012:740987. doi: 10.1155/2012/740987. Epub 2012 Jun 6. Mediators Inflamm. 2012. PMID: 22719179 Free PMC article. Review.
Cited by
-
Neutrophil dynamics in peritoneal carcinomatosis patients treated with cytoreductive surgery and hyperthermic intraperitoneal oxaliplatin.Clin Pharmacokinet. 2013 Dec;52(12):1111-25. doi: 10.1007/s40262-013-0092-3. Clin Pharmacokinet. 2013. PMID: 23828617
-
Attenuation of Chronic Inflammation in Intestinal Organoids with Graphene Oxide-Mediated Tumor Necrosis Factor-α_Small Interfering RNA Delivery.Langmuir. 2024 Feb 7;40(7):3402-13. doi: 10.1021/acs.langmuir.3c02741. Online ahead of print. Langmuir. 2024. PMID: 38325360 Free PMC article.
-
NADPH Oxidase 4-mediated Alveolar Macrophage Recruitment to Lung Attenuates Neutrophilic Inflammation in Staphylococcus aureus Infection.Immune Netw. 2023 Oct 27;23(5):e42. doi: 10.4110/in.2023.23.e42. eCollection 2023 Oct. Immune Netw. 2023. PMID: 37970233 Free PMC article.
-
Dysfunctional nucleus tractus solitarius: its crucial role in promoting neuropathogenetic cascade of Alzheimer's dementia--a novel hypothesis.Neurochem Res. 2012 Apr;37(4):846-68. doi: 10.1007/s11064-011-0680-2. Epub 2012 Jan 5. Neurochem Res. 2012. PMID: 22219130 Review.
-
Tumor necrosis factor-α regulates distinct molecular pathways and gene networks in cultured skeletal muscle cells.PLoS One. 2010 Oct 12;5(10):e13262. doi: 10.1371/journal.pone.0013262. PLoS One. 2010. PMID: 20967264 Free PMC article.
References
-
- Bacon KB, Oppenheim JJ. Chemokines in disease models and pathogenesis. Cytokine Growth Factor Rev. 1998;9:167–173. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

