Cardiac strong inward rectifier potassium channels
- PMID: 19703462
- PMCID: PMC2813336
- DOI: 10.1016/j.yjmcc.2009.08.013
Cardiac strong inward rectifier potassium channels
Abstract
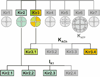
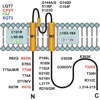
Cardiac I(K1) and I(KACh) are the major potassium currents displaying classical strong inward rectification, a unique property that is critical for their roles in cardiac excitability. In the last 15 years, research on I(K1) and I(KACh) has been propelled by the cloning of the underlying inwardly rectifying potassium (Kir) channels, the discovery of the molecular mechanism of strong rectification and the linking of a number of disorders of cardiac excitability to defects in genes encoding Kir channels. Disease-causing mutations in Kir genes have been shown experimentally to affect one or more of the following channel properties: structure, assembly, trafficking, and regulation, with the ultimate effect of a gain- or a loss-of-function of the channel. It is now established that I(K1) and I(KACh) channels are heterotetramers of Kir2 and Kir3 subunits, respectively. Each homomeric Kir channel has distinct biophysical and regulatory properties, and individual Kir subunits often display different patterns of regional, cellular, and membrane distribution. These differences are thought to underlie important variations in the physiological properties of I(K1) and I(KACh). It has become increasingly clear that the contribution of I(K1) and I(KACh) channels to cardiac electrical activity goes beyond their long recognized role in the stabilization of resting membrane potential and shaping the late phase of action potential repolarization in individual myocytes but extends to being critical elements determining the overall electrical stability of the heart.
Copyright 2009 Elsevier Inc. All rights reserved.
Figures
References
-
- Katz B. Les constantes electriques de la membrane du muscle. Arch Sci Physiol. 1949;2:285–299.
-
- Mascher D, Peper K. Two components of inward current in myocardial muscle fibers. Pflugers Arch. 1969;307(3):190–203. - PubMed
-
- Rougier O, Vassort G, Stampfli R. Voltage clamp experiments on frog atrial heart muscle fibres with the sucrose gap technique. Pflugers Arch Gesamte Physiol Menschen Tiere. 1968;301(2):91–108. - PubMed
-
- Weidmann S. Rectifier properties of Purkinje fibers. Am J Physiol. 1955;183:671.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

