Multifunctional rapidly adapting mechanosensitive enteric neurons (RAMEN) in the myenteric plexus of the guinea pig ileum
- PMID: 19703967
- PMCID: PMC2768021
- DOI: 10.1113/jphysiol.2009.177105
Multifunctional rapidly adapting mechanosensitive enteric neurons (RAMEN) in the myenteric plexus of the guinea pig ileum
Abstract
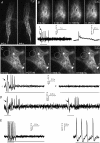
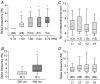
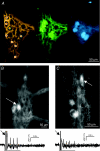
An important feature of the enteric nervous system (ENS) is its capability to respond to mechanical stimulation which, as currently suggested for the guinea-pig ileum, is encoded by specialized intrinsic primary afferent neurons (IPANs). We used von Frey hairs or intraganglionic volume injections to mimic ganglion deformation as observed in freely contracting preparations. Using fast voltage-sensitive dye imaging we identified rapidly adapting mechanosensitive enteric neurons (RAMEN, 25% of all neurons) in the myenteric plexus of the guinea pig ileum. RAMEN responded with phasic spike discharge to dynamic changes during ganglion deformation. This response was reproducible and increased with increasing forces. Deformation-evoked spike discharge was not changed by synaptic blockade with hexamethonium, omega-conotoxin or low Ca(2+)/high Mg(2+), defunctionalization of extrinsic afferents with capsaicin or muscle paralysis with nifedipine, suggesting direct activation of RAMEN. All RAMEN received hexamethonium-sensitive fast EPSPs, which were blocked by omega-conotoxin and low Ca(2+)/high Mg(2+). Seventy-two per cent of RAMEN were cholinergic, 22% nitrergic, and 44% were calbindin and NeuN negative, markers used to identify IPANs. Mechanosensitivity was observed in 31% and 47% of retrogradely traced interneurons and motor neurons, respectively. RAMEN belong to a new population of mechanosensitive neurons which differ from IPANs. We provided evidence for multifunctionality of RAMEN which may fulfil sensory, integrative and motor functions. In light of previously identified mechanosensitive neuron populations, mechanosensitivity appears to be a property of many more enteric neurons than generally assumed. The findings call for a revision of current concepts on sensory transmission within the ENS.
Figures

References
-
- Blackshaw LA, Brookes SJH, Grundy D, Schemann M. Sensory trasmission in the gastrointestinal tract. Neurogastroenterol Motil. 2007;19(suppl. 1):1–19. - PubMed
-
- Brookes S. Retrograde tracing of enteric neuronal pathways. Neurogastroenterol Motil. 2001;13:1–18. - PubMed
-
- Christensen AP, Corey DP. TRP channels in mechanosensation: direct or indirect activation? Nat Rev Neurosci. 2007;8:510–521. - PubMed
-
- Furness JB, Kunze WAA, Bertrand PP, Clerc N, Bornstein JC. Intrinsic primary afferent neurons of the intestine. Prog Neurobiol. 1998;54:1–18. - PubMed
-
- Gershon MD. The enteric nervous system: a second brain. Hosp Pract (Minneap) 1999;34:31–32. 35–38, 41–42. passim. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

