Regulation of protein synthesis by ionizing radiation
- PMID: 19704005
- PMCID: PMC2772731
- DOI: 10.1128/MCB.00711-09
Regulation of protein synthesis by ionizing radiation
Abstract
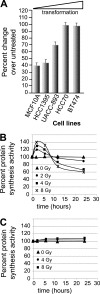
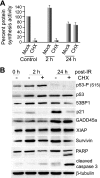
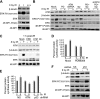
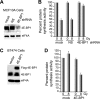
Ionizing radiation (IR) is a physiologically important stress to which cells respond by the activation of multiple signaling pathways. Using a panel of immortalized and transformed breast epithelial cell lines, we demonstrate that IR regulation of protein synthesis occurs in nontransformed cells and is lost with transformation. In nontransformed cells, IR rapidly activates the MAP kinases ERK1/2, resulting in an early transient increase in cap-dependent mRNA translation that involves mTOR and is radioprotective, enhancing the translation of a subset of mRNAs encoding proteins involved in DNA repair and cell survival. Following a transient increase in translation, IR-sensitive (nontransformed) cells inhibit cap-dependent protein synthesis through a mechanism that involves activation of p53, induction of Sestrin 1 and 2 genes, and stimulation of AMP kinase, inhibiting mTOR and hypophosphorylating 4E-BP1. IR is shown to block proteasome-mediated decay of 4E-BP1, increasing its abundance and the sequestration of eIF4E. The IR signal that impairs mTOR-dependent protein synthesis at late times is assembly of the DNA damage response machinery, consisting of Mre11, Rad50, and NBS1 (MRN); activation of the MRN complex kinase ATM; and p53. These results link genotoxic signaling from the DNA damage response complex to the control of protein synthesis.
Figures




References
-
- Abedin, M. J., D. Wang, M. A. McDonnell, U. Lehmann, and A. Kelekar. 2007. Autophagy delays apoptotic death in breast cancer cells following DNA damage. Cell Death Differ. 14:500-510. - PubMed
-
- Albert, J. M., K. W. Kim, C. Cao, and B. Lu. 2006. Targeting the Akt/mammalian target of rapamycin pathway for radiosensitization of breast cancer. Mol. Cancer Ther. 5:1183-1189. - PubMed
-
- Bhandari, B. K., D. Feliers, S. Duraisamy, J. L. Stewart, A. C. Gingras, H. E. Abboud, G. G. Choudhury, N. Sonenberg, and B. S. Kasinath. 2001. Insulin regulation of protein translation repressor 4E-BP1, an eIF4E-binding protein, in renal epithelial cells. Kidney Int. 59:866-875. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
